��Ŀ����
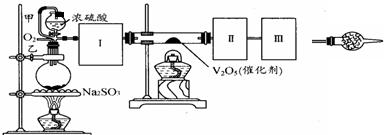
����ʵ����ҩƷ�����淽�������������ơ���װ������ʹ������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
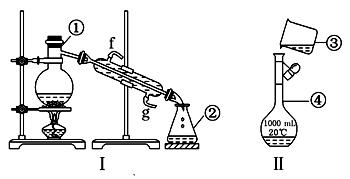
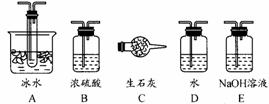
��1��KMnO4ͨ����������ɫ�Լ�ƿ�У������Լ���������ɫ�Լ�ƿ������ǣ�����ĸ���ţ� ��
a��Ũ���� b. ��ˮ c. �ռ���Һ
��2��д���������������ƣ����������� ������������ .
��3��������װ�â�������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ�����������������������������������е�ʵ�����������Ϊ��������
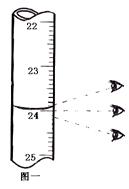
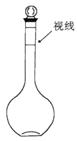
��4����������250 mL 0.2 mol/L NaCl��Һ��װ�â���ijͬѧת����Һ��ʾ��ͼ��ͼ�еĴ��ڵĴ����ǣ�
�� ���� ��

��1��KMnO4ͨ����������ɫ�Լ�ƿ�У������Լ���������ɫ�Լ�ƿ������ǣ�����ĸ���ţ� ��
a��Ũ���� b. ��ˮ c. �ռ���Һ
��2��д���������������ƣ����������� ������������ .
��3��������װ�â�������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ�����������������������������������е�ʵ�����������Ϊ��������
��4����������250 mL 0.2 mol/L NaCl��Һ��װ�â���ijͬѧת����Һ��ʾ��ͼ��ͼ�еĴ��ڵĴ����ǣ�
�� ���� ��
��14�֣�
��1��c
��2����������ƿ ������ƿ
��3���¶ȼ� ����
��4����תҺʱû���ò����������� ��ѡ�õ�����ƿ��ԣ�Ӧ����250mL������ƿ��
��1��c
��2����������ƿ ������ƿ
��3���¶ȼ� ����
��4����תҺʱû���ò����������� ��ѡ�õ�����ƿ��ԣ�Ӧ����250mL������ƿ��
��

��ϰ��ϵ�д�
�����Ŀ