��Ŀ����
ij�ռ���Ʒ�к����������������õĿ��������ʣ�Ϊ�˲ⶨ�䴿�ȣ��������µζ�������
E������ƿ�µ�һ�Ű�ֽ���ζ����յ㣬��¼�յ�̶�ΪV2 mL��
�ش��������⣺
��1����ȷ���������˳����(����ĸ��д)
 D
D  ��
��
��2������E�е���ƿ�µ�һ�Ű�ֽ�������� ��
��3������D��Һ��Ӧ������ ��
��4���ζ��յ�������� ��
��5������ʽ�ζ���û���ñ�H2SO4��ϴ����Բⶨ����к�Ӱ��? (�ƫ�ߡ�����ƫ�͡�����Ӱ�족��������������ȷ)��
��6�����ռ���Ʒ�Ĵ��ȼ���ʽ�� ��
| A����250mL����ƿ�ж��ݳ�250mL�ռ���Һ�� |
| B���ü�ʽ�ζ�����ȡ25mL�ռ���Һ����ƿ�в��μӼ��μ�����ָʾ���� |
| C������ƽ��ȷ��ȡ�ռ���Ʒmg�����ձ��м�����ˮ�ܽ⣻ |
| D�������ʵ���Ũ��ΪC mol��L-1�ı�H2SO4��Һװ����ʽ�ζ��ܣ�����Һ�棬���¿�ʼ�̶�ΪV1mL�� |
�ش��������⣺
��1����ȷ���������˳����(����ĸ��д)
 D
D 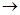 ��
����2������E�е���ƿ�µ�һ�Ű�ֽ�������� ��
��3������D��Һ��Ӧ������ ��
��4���ζ��յ�������� ��
��5������ʽ�ζ���û���ñ�H2SO4��ϴ����Բⶨ����к�Ӱ��? (�ƫ�ߡ�����ƫ�͡�����Ӱ�족��������������ȷ)��
��6�����ռ���Ʒ�Ĵ��ȼ���ʽ�� ��
��1����C��A��B��D��E��
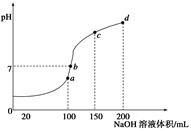
��2������ȷ�ж��յ�ʱ��ɫ�ı仯�����
��3����̶Ȼ������µ�ijһ�̶�
��4���ɻ�ɫ��Ϊ��ɫ
��5��ƫ��
��6��80(V2-V1)c/m%
��2������ȷ�ж��յ�ʱ��ɫ�ı仯�����
��3����̶Ȼ������µ�ijһ�̶�
��4���ɻ�ɫ��Ϊ��ɫ
��5��ƫ��
��6��80(V2-V1)c/m%
�����������1����ʵ��ʱӦ�ȳ���һ�������Ĺ��壬�ܽ�����Ƴ���Һ����ȡ����Һ����ƿ�У�Ȼ���ñ�Һ���еζ����ʴ�Ϊ��C��A��B��D��E��
��2������ƿ�µ�һ�Ű�ֽʹ�ζ��յ���ɫ�仯�����ԣ����ڷֱ棬�ʴ�Ϊ������ȷ�ж��յ�ʱ��ɫ�ı仯�����
��3���ζ���0�̶����ϣ��ζ�ǰӦ���ڵ���̶Ȼ������µ�ijһ�̶ȣ�Ϊ��С�����첿��Ӧ����Һ�壬�����ݣ�
��4�� ָʾ��Ϊ���ȣ���ɫ��ΧΪ 3.1��4.4���յ�ʱpHԼΪ4.4����ɫ�ɻ�ɫ��Ϊ��ɫ��
�ʴ�Ϊ���ɻ�ɫ��Ϊ��ɫ��
��5�� ���ҺŨ�ȱ�С��������ʴ�Ϊ��ƫ��
��6�� ��������ʵ���Ϊc��V2-V1����10-3mol����n��NaOH��=2c��V2-V1����10-3mol��m��NaOH��=80c��V2-V1����10-3������Ʒ��NaOH������Ϊ10��80c��V2-V1����10-3��
�����ռ���Ʒ����Ϊ10��80��(V2-V1) ��10-3c/m��100%=80(V2-V1) c/m%��ע�⣺VΪmL����

��ϰ��ϵ�д�
 ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�
�����Ŀ