��Ŀ����
����Ŀ��������ʵ���Ũ�ȵ�ZnSO4��CuSO4�Ļ����Һ�У���μ���1.5 mol��L1��Na2S��Һֱ����Һ������������Ȼ����ˡ���֪��Ksp(ZnS)=2.0��1022��Ksp(CuS)=1.3��1036������˵������ȷ����
A.������CuS������ZnS���������ڳ�����ת��
B.��ZnS������ȫʱ����Һ��![]() =6.5��1015
=6.5��1015
C.���˵õ��ij�����CuS��ZnS�Ļ����
D.���˺�õ�����Һ�����м�������Cu2+��Zn2+
���𰸡�A
��������
A. �����ʵ���Ũ�ȵ�ZnSO4��CuSO4�Ļ����Һ��KspС���ȳ�����
B. ��ZnS������ȫʱ����Һ�� =
= ��
��
C. ��μ���1.5 mol��L1��Na2S��Һֱ����Һ��������������֪Cu2+��Zn2+��ת��Ϊ������
D. ���ܵ���ʴ����ܽ�ƽ�⣬������Ũ��Ϊ1��105mol/Lʱ��Ϊ������ȫ������
A. �����ʵ���Ũ�ȵ�ZnSO4��CuSO4�Ļ����Һ��KspС���ȳ�������Ϊ���������ɣ�������������ת����A����������⣻
B. ��ZnS������ȫʱ����Һ�� =
= =
=![]() =6.5��10-15��B����ȷ�����������⣻
=6.5��10-15��B����ȷ�����������⣻
C. ��μ���1.5 mol��L1��Na2S��Һֱ����Һ��������������֪Cu2+��Zn2+��ת��Ϊ���������˺�õ��ij�����CuS��ZnS��C����ȷ�����������⣻
D. ���ܵ���ʴ����ܽ�ƽ�⣬������Ũ��Ϊ1��105mol/Lʱ��Ϊ������ȫ���������˺���Һ�����м�������Cu2+��Zn2+��D����ȷ�����������⣻
��ѡA��

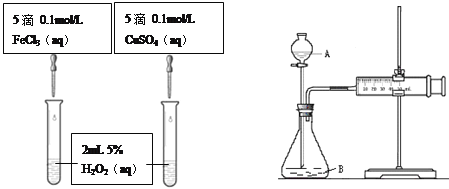
����Ŀ��������H2O2�ڷ�Ӧʱ��������Ⱦ�ﱻ��Ϊ��ɫ������������ܵ�����Խ��Խ��Ĺ�ע��
I.ijʵ��С����H2O2�ֽ�Ϊ����̽��Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�졣�ڳ����°������±���ʾ�ķ������ʵ�顣
ʵ���� | ��Ӧ�� | ���� |
�� | 10 mL 2% H2O2��Һ | �� |
�� | 10 mL 5% H2O2��Һ | �� |
�� | 10 mL 5% H2O2��Һ | 1 mL 0.1 mol��L-1FeCl3��Һ |
�� | 10 mL 5% H2O2��Һ+����HCl��Һ | 1 mL 0.1 mol��L-1FeCl3��Һ |
�� | 10 mL 5% H2O2��Һ+����NaOH��Һ | 1 mL 0.1 mol��L-1FeCl3��Һ |
��1��ʵ��ٺ͢ڵ�Ŀ����_________________________________��ͬѧ�ǽ���ʵ��ʱû�й۲쵽������������ó����ۡ�������ʾ��ͨ��������H2O2�ȶ������ֽ⡣Ϊ�˴ﵽʵ��Ŀ�ģ����ԭʵ�鷽���ĸĽ�������_________________________����һ�ַ������ɣ���
��2��ʵ��ۢܢ��У�������������������ʱ��仯�Ĺ�ϵ��ͼ��ʾ��
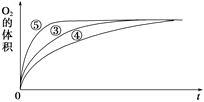
������ͼ�ܹ��ó���ʵ�������________________________________��
II.������ʾ��ijЩ�������ӻ�����������H2O2�ķֽ�������á�Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч������ʵ��С���ͬѧ���������ͼ��ʾ��ʵ��װ�ý���ʵ�顣
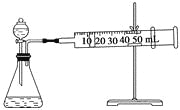
��1��ijͬѧͨ���ⶨO2��������Ƚ�H2O2�ķֽ����ʿ�����ʵ��ʱ����ͨ������_______��______���Ƚϡ�
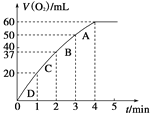
��2��0.1 g MnO2��ĩ����50 mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ������ͻ�ѧ��Ӧ���ʱ仯��ԭ��_____________�������H2O2�ij�ʼ���ʵ���Ũ��Ϊ________________(������λ��Ч����)��
Ϊ̽��MnO2�ڴ�ʵ���ж�H2O2�ķֽ�������ã��貹������ʵ�飨�� ��д�������������/span>a._________________��b.___________________��
����Ŀ��NiCl2�ǻ����ϳ�������Ҫ����Դ����ҵ�����ú���(Ni)�ϴ�������Ҫ����Ni��������SiO2��Al2O3��Fe�������������ᡢ������ʣ������Ȼ������壨NiCl2��nH2O���������£�
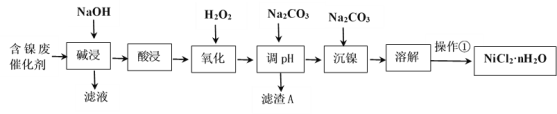
���ֽ�����������Ksp����ֵ���±���ʾ��
��ѧʽ | Fe(OH)2 | Fe(OH)3 | Al(OH)3 | Ni(OH)2 |
Ksp����ֵ | 10-17 | 10-38 | 10-34 | 10-15 |
�ش��������⣺
��1��Ni��ԭ������Ϊ28��λ��Ԫ�����ڱ��������ڣ�����������Ϊ2������ԭ�ӽṹʾ��ͼΪ________________��
��2��������������ܽ�����ʹ�õ���Ϊ_____________���������ʱ������Ӧ�����ӷ���ʽΪSiO2+2OH-=SiO32-+H2O��___________________��
��3��������������H2O2��Һ����������____________________(�����ӷ���ʽ��ʾ)��Ȼ�����pHʹ��Һ����Ԫ��ǡ����ȫ����������Ũ����10-5mol��L-1ʱ�����ӳ�����ȫ������ʱ�����µ�pHԼΪ____________��
��4��������������ʵ���������Ϊ�������ȣ�Ũ����_________________________Ϊֹ����ȴ�ᾧ�����ˡ�ϴ�ӡ�������ò�Ʒ��
��5��Ϊ�˲ⶨNiCl2nH2O�нᾧˮ��Ŀ��������ʵ�飺ȡ23.8 g��Ʒ��һ����������ˮ��13.0 g NiCl2����n=__________��
��6���������ѳ�Ϊ��϶�����������Ҫ������ͣ����ڼ��Ե������Һ�Ĺ���ԭ�����£�M+Ni(OH)2![]() MH+NiOOH(ʽ��MΪ����Ͻ�)��д����س������������ĵ缫��Ӧʽ__________________��
MH+NiOOH(ʽ��MΪ����Ͻ�)��д����س������������ĵ缫��Ӧʽ__________________��