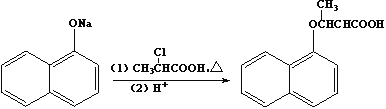
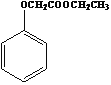
��Ŀ����
����Ŀ�������������Ԫ��֮һ������ֲ�ﺬ�зḻ�ĵ�Ԫ�ء���ʵ���У��Ӻ�������ȡ����������£�

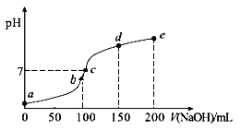
(1)��ͼΪ�����Ƶ������ͼ����������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ��������_____________________��������������ѡ��������������ñ����ĸ��д����
A.�ձ� B.���� C.������ D.������ E.�ƾ��� F.������
(2)����۵�����Ϊ_____________����������貣���������ձ����___________________��
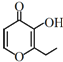
(3)�����������������������ѡ��μ�ϡ���ᣬ�ټ���H2O2�ķ�������Ӧ�����ӷ���ʽΪ______________________________________��
(4)����ݣ���ѡ����л��Լ���___________��
A���ױ����ƾ� B�����Ȼ�̼���� C�����͡����� D�����͡�����
(5)��������ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ�ѧ����Ϊ_____________________��
���𰸡�BDE ���� ��Һ©�� 2I-+H2O2+2H+ ==I2+2H2O B ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ��
��������
�����̿���֪�����������պ��ܽ⣬Ȼ��۹��˵õ��������ӵ���Һ���ܷ���2I-+Cl2�TI2+ Cl-����Ϊ��ȡ�õ�����ı���Һ����Ϊ����õ����ʵ���
��1��������Ҫ�����������ǡ����Ǽݡ��ƾ��ơ�����ǯ�����������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ��������B.������D.��������E.�ƾ��ƣ���ˣ�������ȷ���ǣ�BDE��
(2)������Ƿ��뺣���ҵ�����Һ�������ù��˵ķ������롣�����Ϊ�ӵ�ˮ����ȡ�⣬����ȡ�ķ������롣��ȡ���貣���������ձ�����Һ©�����𰸣���������Һ©����
(3)�������������������������ȡ�ⵥ�ʣ�������˫��ˮ�����õμ�ϡ���ᣬ��Ӧ�����ӷ���ʽΪ2I-+H2O2+2H+ ==I2+2H2O���𰸣�2I-+H2O2+2H+ ==I2+2H2O��
(4)�����Ϊ�ӵ�ˮ����ȡ�⡣��ȡ����ѡ��ԭ���Ǻ�ˮ�������ܣ�Ҫ��ȡ�����������е��ܽ�ȴ�����ˮ�е��ܽ�ȣ����Կ���ѡ�����Ȼ�̼��A���ƾ���ˮ���ܣ�B�������Ȼ�̼����������C�������ˮ���ܣ�D�����ͺ�ˮ���ܣ��𰸣�B��
(5)��������ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ�ѧ����Ϊ������ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ���ɡ������������˵�����е��ʵ⣩������𰸣�ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ��