��Ŀ����
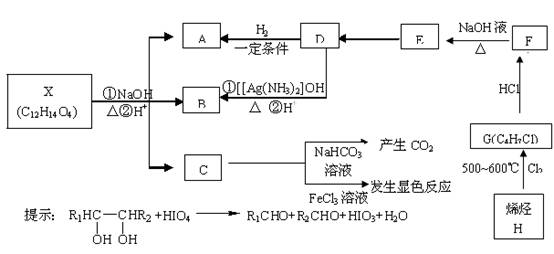
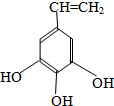
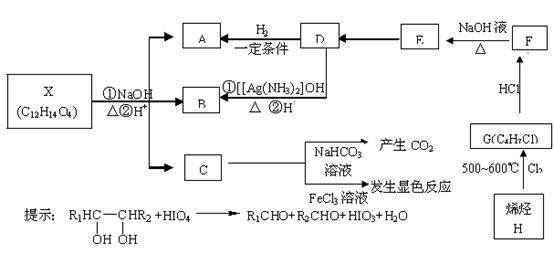
��16�֣�.�л���X��A��B��C��D��E��F��G��H���Է�������ת��������C�����ϵ�һ�ȴ���ֻ�����֣�ϩ��H��HCl�ӳ����ɵIJ��������֣�D���������е�ԭ�Ӳ����ܾ�����ͬһƽ���ڡ�
�ش��������⣺
��1��C�к��������ŵ����ƣߣߣߣߣߣߣߣߣߣߣߣߣ�
��2��д���������ʵĽṹ��ʽ��
D�ߣߣߣߣߣߣߣߣߣߣߣߡ�X�ߣߣߣߣߣߣߣߣߣߣߣߣ�
��3��д�����л�ѧ��Ӧ����ʽ����Ӧ����
��H G���ߣߣߣߣߣߣߣߣߣߣߣߣ���Ӧ���ͣ��ߣߣߣߣߣ�
G���ߣߣߣߣߣߣߣߣߣߣߣߣ���Ӧ���ͣ��ߣߣߣߣߣ�
��F E���ߣߣߣߣߣߣߣߣߣߣߣߣ���Ӧ���ͣ��ߣߣߣߣߣ�
E���ߣߣߣߣߣߣߣߣߣߣߣߣ���Ӧ���ͣ��ߣߣߣߣߣ�
��4��C����һ��ͬ���칹�����������������
�����ڷ����廯����ڱ����ϵ�һ��ȡ����ֻ��һ�֣�����������NaOH��Һ��ȫ��Ӧ1 mol������3 mol NaOH��д�������еĽṹ��ʽ���ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
���𰸡�
CH3CH2CH=CH2 �� Cl2  CH3CH(Cl)CH=CH2 ��HCl��3�֣� ȡ����Ӧ
CH3CH(Cl)CH=CH2 ��HCl��3�֣� ȡ����Ӧ
��16�֣���1�����ǻ����ǻ��� �Ȼ���2�֣�
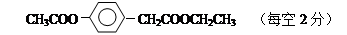 ��2��CH3CHO
��2��CH3CHO
(3)
|
 CH3CH(Cl)CH=CH2 ��HCl��3�֣� ȡ����Ӧ
CH3CH(Cl)CH=CH2 ��HCl��3�֣� ȡ����Ӧ
CH3CH(Cl)CH(Cl)CH3 �� 2NaOH 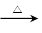 CH3CH(OH)CH(OH)CH3
��2NaCl��3�֣�ˮ�ⷴӦ��ȡ����Ӧ
CH3CH(OH)CH(OH)CH3
��2NaCl��3�֣�ˮ�ⷴӦ��ȡ����Ӧ
(4)
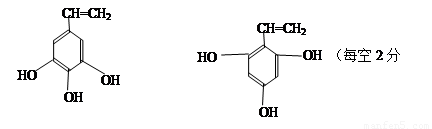
��������

��ϰ��ϵ�д�
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
�����Ŀ



 ��
��

 +HIO4��R1CHO+R2CHO+HIO3+H2O
+HIO4��R1CHO+R2CHO+HIO3+H2O
 G���ߣߣߣߣߣߣߣߣߣߣߣߣ���Ӧ���ͣ��ߣߣߣߣߣ�
G���ߣߣߣߣߣߣߣߣߣߣߣߣ���Ӧ���ͣ��ߣߣߣߣߣ�