��Ŀ����
�±��е��������ƻ�1 mol�����еĻ�ѧ�������ĵ�����(kJ):
�����������ݻش�(1)~(5)�⡣
(1)�������ʱ������е�������͵�������������
A.H2 B.Cl2 C.Br2 D.I2
(2)�����⻯����,���ȶ���������������
A.HCl����������B.HBr����������C.HI
(3)X2+H2 2HX(X����Cl��Br��I )�ķ�Ӧ�����ȷ�Ӧ���Ƿ��ȷ�Ӧ?��:��______________��
2HX(X����Cl��Br��I )�ķ�Ӧ�����ȷ�Ӧ���Ƿ��ȷ�Ӧ?��:��______________��
(4)��ͬ������,X2(X����Cl��Br��I)�ֱ���������Ӧ,�����ĵ����ʵ���������ʱ,�ų������յ�����������������������
(5)�����ϱ��е�����,������ȷ�ش������(4)��?
��:����������,��ĸ�������______________________��
| ���� | Cl2 | Br2 | I2 | HCl | HBr | HI | H2 |
| ����/kJ | 243 | 193 | 151 | 432 | 366 | 298 | 436 |
�����������ݻش�(1)~(5)�⡣
(1)�������ʱ������е�������͵�������������
A.H2 B.Cl2 C.Br2 D.I2
(2)�����⻯����,���ȶ���������������
A.HCl����������B.HBr����������C.HI
(3)X2+H2
 2HX(X����Cl��Br��I )�ķ�Ӧ�����ȷ�Ӧ���Ƿ��ȷ�Ӧ?��:��______________��
2HX(X����Cl��Br��I )�ķ�Ӧ�����ȷ�Ӧ���Ƿ��ȷ�Ӧ?��:��______________�� (4)��ͬ������,X2(X����Cl��Br��I)�ֱ���������Ӧ,�����ĵ����ʵ���������ʱ,�ų������յ�����������������������
(5)�����ϱ��е�����,������ȷ�ش������(4)��?
��:����������,��ĸ�������______________________��
(1)A(2)A(3)���ȷ�Ӧ(4)Cl2
(5)�ܡ�Ԫ�صķǽ�����Խǿ,���ɵ��⻯��Խ�ȶ�,��Ӧ�ų���������Խ��
(5)�ܡ�Ԫ�صķǽ�����Խǿ,���ɵ��⻯��Խ�ȶ�,��Ӧ�ų���������Խ��
�ƻ�1 mol���ʵĻ�ѧ��ʱ�����ĵ���������ͬ���������γɸ����ʵ�ԭ���γ�1 mol�����ʵĻ�ѧ���ų����������;�ų�������Խ��,���ʱ������е�����Խ��,����Խ�ȶ���(1)����1 mol H2ʱ�ų����������,Ϊ436 kJ;(2)���⻯����,����1 mol HClʱ�ų����������,Ϊ432 kJ;(3)�ֱ�����������Ӧ�ų�����������Ϊ:185 kJ��103 kJ��9 kJ��

��ϰ��ϵ�д�
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
�����Ŀ
 2NH3(g) + Q kJ(Q��0)������˵���У���ȷ����
2NH3(g) + Q kJ(Q��0)������˵���У���ȷ����

 C(g)��D(g)�����е������仯��ͼ��ʾ������˵����ȷ���ǣ� ��
C(g)��D(g)�����е������仯��ͼ��ʾ������˵����ȷ���ǣ� ��
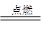 2CO2��2H2��O2
2CO2��2H2��O2 CO2��2H2O
CO2��2H2O 3CO2��4H2O������˵���в���ȷ���ǣ� ��
3CO2��4H2O������˵���в���ȷ���ǣ� ��