��Ŀ����
��15�֣���Ԫ���뵪Ԫ�ؿ���ɶ���������NH3��NH4+��N2H4�ȡ�
I.��1������������H2O��H2O2��CH4��C2H6�ķ��ӽṹ��д����N2H4����ʽ�ߣߣߣߣߡ�
��2��ij��N2H5Cl��NH4Cl���ƣ��ǿ�����ˮ�����ӻ��������Һ��ˮ����������ԡ�N2H5Cl��Һ������ԭ�������ӷ���ʽ��ʾ���ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��3�����������ʣ�NH3��Mn2O3��ZnCl2��MnO2��NH4Cl��Zn��H2O����п���̵����������ԭ��Ӧ��ijЩ��Ӧ�NH4ClΪ����֮һ����ijЩ�����NH3Ϊ����֮һ����
д��������ѧ��Ӧ����ʽ���ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ� II.�ں��������£���ʼ�ݻ���Ϊ5L�ļס������ܱ������У���Ϊ����������Ϊ��ѹ�������������з�Ӧ��N2+3H2 2NH3���й����ݼ�ƽ��״̬�ص���±���
II.�ں��������£���ʼ�ݻ���Ϊ5L�ļס������ܱ������У���Ϊ����������Ϊ��ѹ�������������з�Ӧ��N2+3H2 2NH3���й����ݼ�ƽ��״̬�ص���±���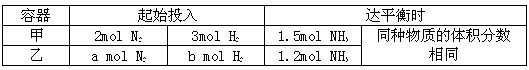
��4�������ܱ���������һ����ƽ��״̬���ǣߣߣߣߣߣ�����ĸ��
| A�������ڵĻ�������ƽ����Է����������ٱ仯 | |
| B�������ڵĵ�Ԫ�ص��������ٱ仯 | |
C�����������������백������������֮��Ϊ2��3 | D���γ�1mol N N����ͬʱ�γ�6molN��H�� |
��6����ʼʱ����������������ѹǿ�ģߣߣߣߣ߱���
��15�֣�
��1��  (2��)
(2��)
��2��N2H5����H2O N2H4��H2O��H�� (2��)
N2H4��H2O��H�� (2��)
��3��Zn��2MnO2��2 NH4Cl ��ZnCl2 ��Mn2O3��2NH3����H2O (3��)
��4��AD��2�֣� ��5��37.5% (3��) ��6��0.8 (3��)
����

 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д���Ԫ���뵪Ԫ�ؿ���ɶ���������NH3��NH4+��N2H4�ȡ�
��1������������H2O��H2O2��CH4��C2H6�ķ��ӽṹ��д����N2H4����ʽ______��
��2��ij��N2H5Cl��NH4Cl���ƣ��ǿ�����ˮ�����ӻ��������Һ��ˮ����������ԡ�N2H5Cl��Һ������ԭ�������ӷ���ʽ��ʾ�� ��
��3�����������ʣ�NH3��Mn2O3��ZnCl2��MnO2��NH4Cl��Zn��H2O����п���̵����������ԭ��Ӧ��ijЩ��Ӧ�NH4ClΪ����֮һ����ijЩ�����NH3Ϊ����֮һ����д��������ѧ��Ӧ����ʽ��_____________________________________________________��
���ں��������£���ʼʱ�ݻ���Ϊ5 L�ļס������ܱ������У���Ϊ���������� ��Ϊ��ѹ�������������з�Ӧ��N2+3H2![]() 2NH3���й����ݼ�ƽ��״̬�ص���±���
2NH3���й����ݼ�ƽ��״̬�ص���±���
| ���� | ��ʼͶ�� | ��ƽ��ʱ | ||
| �� | 2 mol N2 | 3 mol H2 | 1.5 mol NH3 | ͬ�����ʵ����������ͬ |
| �� | a mol N2 | b mol H2 | 1.2 mo l NH3 | |
��4�������ܱ���������һ����ƽ��״̬����____________(����ĸ)
A�������ڵĻ�������ƽ����Է����������ٱ仯
B�������ڵĵ�Ԫ�ص��������ٱ仯
C�����������������백������������֮��Ϊ2��3
D���γ�1 mol N��N����ͬʱ�γ�6 mol N��H��
��5���������е�����ת����Ϊ ��
��6����ʼʱ����������������ѹǿ��_________����