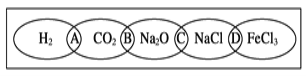
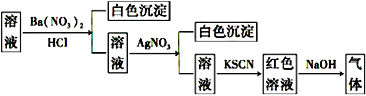
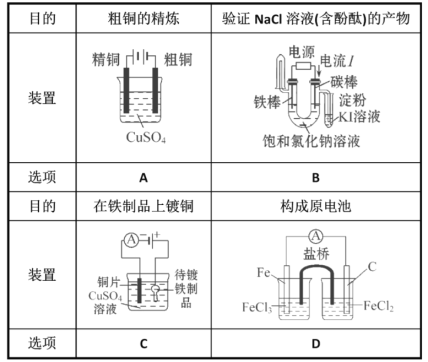
��Ŀ����
����Ŀ��ʵ������Na2CO3��10H2O��������240 mL 0.1 mol��L��1 Na2CO3��Һ���ش��������⣺
��1��ʵ������������������ƽ���ձ����������ͽ�ͷ�ι��⣬����Ҫһ����Ҫ����X��������X��������__________��
��2����������ƽ��ȡNa2CO3��10H2O������Ϊ_____________________��
��3������ʱ����ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸֻ����һ�Σ�______________ED��
A��������ˮϴ���ձ���������2��3�Σ�ϴ��Һ��ע������X����
B����������ƽ���������Na2CO3��10H2O���壬�����ձ��У��ټ�������ˮ���ò���������������ʹ����ȫ�ܽ�
C��������ȴ��Na2CO3��Һ�ز�����ע������X��
D��������X�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ�����͵�ǡ����̶�������
F������������X��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1~2 cm��
��4�������������������ʹ������Һ��Ũ��ƫ�ߵ���______________��
��û�н���A����
�ڳ���ʱ��Na2CO3��10H2O��ʧȥ���ֽᾧˮ
�۽���E����ʱ����
��D������ɺ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�ˮ���̶�
�ݳ���ʱ��������������IJ������г�����
����Һδ��ȴ����ת�Ƶ�����ƿ�С�
���𰸡�250mL����ƿ 7.2g BCAF �ڢۢ�
��������
������Ҫ����ʵ���������������
��1���������Ʋ����Ǽ��㡢�������ܽ⡢ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ����������������ƿ���������Һ���ѡ���������ƿ��
��2������n=cV��������Na2CO3�����ʵ���������Na2CO3��10H2O�����ʵ�������Na2CO3�����ʵ���������![]() ����Na2CO3��10H2O��������
����Na2CO3��10H2O��������
��3����������һ�����ʵ���Ũ����Һ�Ļ�������˳���������
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����![]() ������������
������������
(1)���������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���,һ����������ƽ����,��ҩ��ȡ��ҩƷ,���ձ����ܽ�(������Ͳ��ȡˮ�����ձ�),���ò���������,�����ܽ⡣��ȴ��ת�Ƶ�����ƿ��,���ò���������,ϴ���ձ���������23��,����ϴ��Һ��������ƿ��,��ˮ��Һ�����̶���12cmʱ,���ý�ͷ�ιܵμ�,����ݵߵ�ҡ�ȡ���������������������ƽ���ձ���������������ƿ����ͷ�ι�,����![]() ��Һ��Ӧѡ��250mL����ƿ�����������������250mL����ƿ��
��Һ��Ӧѡ��250mL����ƿ�����������������250mL����ƿ��
�ʴ�Ϊ��250mL����ƿ��
(2)������240ml������ƿ,��ѡ��250ml������ƿ,������Һ�����Ϊ250ml,�����Na2CO3�����ʵ���![]() ��Na2CO3��10H2O�����ʵ�������Na2CO3�����ʵ���,����Na2CO3��10H2O������
��Na2CO3��10H2O�����ʵ�������Na2CO3�����ʵ���,����Na2CO3��10H2O������![]() ��
��
�ʴ�Ϊ��7.2g��
(3)���������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȿ�֪��ȷ�IJ���˳��Ϊ��BCAFED��
�ʴ�Ϊ��BCAF��
(4)��û�н���A�������������ʲ�����ģ����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ��ʲ�ѡ��
�ڳ���ʱ, Na2CO3��10H2O��ʧȥ���ֽᾧˮ�����ȡ�Ĺ����к������ʵ����ʵ���ƫ����ҺŨ��ƫ�ߣ���ѡ��
�۽���E����ʱ���ӣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���ѡ��
��D������ɺ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ��ʲ�ѡ��
�ݳ���ʱ��������������IJ������г���,���³�ȡ��������ƫС��Ũ��ƫ�͡��ʲ�ѡ��
����Һδ��ȴ����ת�Ƶ�����ƿ�У�����Һ��������������Һ���ƫС��Ũ��ƫ��ѡ��
��ѡ���ڢۢޡ�

 ��У����ϵ�д�
��У����ϵ�д�