��Ŀ����
����Ŀ��(1)��2L���ܱ������з���4mol N2O5���������·�Ӧ��2N2O5(g)![]() 4NO2(g)+O2(g)����Ӧ��5minʱ�����N2O5ת����20%����v(NO2)Ϊ_________��5minʱ��N2O5�ڻ�������е����������____��
4NO2(g)+O2(g)����Ӧ��5minʱ�����N2O5ת����20%����v(NO2)Ϊ_________��5minʱ��N2O5�ڻ�������е����������____��
(2)ij�¶�ʱ����һ��2L���ܱ���������X��Y��Z�������ʵ����ʵ�����ʱ��仯��������ͼ��ʾ������ͼ�����������

�ٸ÷�Ӧ�Ļ�ѧ����ʽΪ______��
����X��Y��Z��Ϊ������2minʱ��Ӧ�ﵽƽ������ʱ��ϵ��ѹǿ�뿪ʼʱ��ѹǿ֮��Ϊ____��
����X��Y��Z��Ϊ���������ƽ��ʱ�������ڻ�������ƽ����Է�����������ʼͶ��ʱ__(��������������С�����������)��
���𰸡�0.16 mol��L-1��min-1 61.5% 3X+Y![]() 2Z 9��10 ����
2Z 9��10 ����
��������
������Ҫ���컯ѧƽ�⡣
(1) N2O5ת����20%������n(N2O5)=4mol��20%=0.8mol����ϵ�и���ֵ����ʵ������±���ʾ����λ��mol����
2N2O5 | 4NO2 | O2(g) | |
2 | 4 | 1 | |
�� | 4 | 0 | 0 |
ת | 0.8 | 1.6 | 0.4 |
ƽ | 3.2 | 1.6 | 0.4 |
��n(NO2)=1.6mol��v(NO2)=![]() = 0.16 mol��L-1��min-1�����ݰ���٤�����ɿɵã�N2O5�ڻ�������е����������
= 0.16 mol��L-1��min-1�����ݰ���٤�����ɿɵã�N2O5�ڻ�������е����������![]() =61.5%��
=61.5%��
(2)�ٷ�Ӧ�У�X��Y�����ʵ�����С��Z�����ʵ������࣬��X��YΪ��Ӧ�ZΪ�����0-2min�ڣ���n(X)=0.3mol����n(Y)=0.1mol����n(Z)=0.2mol����2min�����ֵ����ʵ������ٸı䣻���ԣ��÷�Ӧ�Ļ�ѧ����ʽΪ3X+Y![]() 2Z��
2Z��
�ڸ�����������״̬����:PV=nRT��2minʱ��ϵѹǿ�뿪ʼʱ��ѹǿ֮�ȵ�������ʱ�������������ʵ���֮�ȣ��ñ���Ϊ��0.9+0.7+0.2������1.0+1.0��=1.8��2=9��10��
�۸÷�Ӧ�Ļ�ѧ����ʽΪ3X+Y![]() 2Z����Ӧ������Ӧ��ʼ������ϵ�������ʵ�����С��������ϵ�и���ֶ������壬����ϵ�����������䣬��������ƽ����Է�����������
2Z����Ӧ������Ӧ��ʼ������ϵ�������ʵ�����С��������ϵ�и���ֶ������壬����ϵ�����������䣬��������ƽ����Է�����������

����Ŀ��Fe(OH)3�㷺Ӧ����ҽҩ�Ƽ�������������������Ʊ����輰װ�����£���������ƿ�м���16.7gFeSO4��7H2O��40.0ml����ˮ���߽����������3.0mLŨH2SO4���ټ���2.0gNaClO3���塣ˮԡ������80�棬����һ��ʱ�����NaOH��Һ����ַ�Ӧ�������ˡ�ϴ�ӡ�����ò�Ʒ��
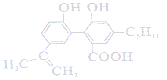
(1)NaClO3����FeSO4��7H2O�����ӷ���ʽΪ_____________��
(2)����Ũ���������Ϊ_________(����)��
a.�ṩ���Ի�������ǿNaClO3������ b.��ȥFeSO4��7H2O�Ľᾧˮ
c.����Fe3+ˮ�� d.��Ϊ������
(3)����Fe2+�Ѿ���ȫ��������ʹ�õ��Լ���_________��
(4)�о���ͬʱ�����¶���NaClO3������Fe2+����Ч����Ӱ�죬��ƶԱ�ʵ�����±�
��� | T/�� | FeSO4��7H2O/g | NaClO3/g | ������/% |
i | 70 | 25 | 1.6 | a |
ii | 70 | 25 | m | b |
iii | 80 | n | 2.0 | c |
iv | 80 | 25 | 1.6 | 87.8 |
��m=______��n=______��
����c>87.8>a����a��b��c�Ĵ�С��ϵΪ___________��
(5)����NaOH��Һ�Ʊ�Fe(OH)3�Ĺ����У�������ˮԡ�¶ȣ�Fe(OH)3�IJ����½�����ԭ����___
(6)�ж�Fe(OH)3����ϴ�Ӹɾ���ʵ�����Ϊ_________________��
(7)���ʵ��֤���ƵõIJ�Ʒ��FeOOH(���費����������)��___________��