��Ŀ����
��14�֣���������(ClNO)���л��ϳ��е���Ҫ�Լ�������NO��Cl2��ͨ�������·�Ӧ�õ������������й��������£�
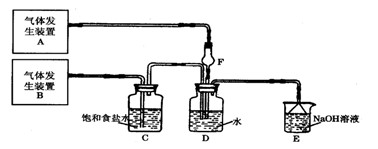
ij�о���ѧϰС���������������������ͨ�������ȡ�������ȣ��������ͼ��ʾʵ��װ�á�ʵ�鿪ʼǰK2���ڴ�״̬��K1��K3���ѹرա���
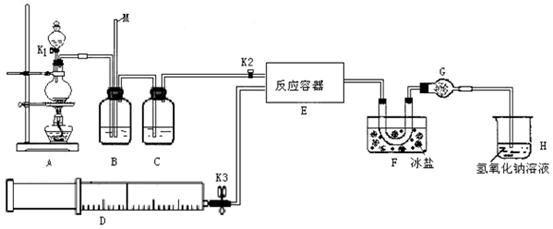
�Իش��������⣺
��1������D��װ�д�����NO���壬��B��Ӧѡ�õ��Լ�Ϊ ��ʵ��ʱ����B��ȥijЩ�������岢ͨ���۲�B�е��������жϷ�Ӧ���ʣ�B�����е������� ������D��װ�д�������������C��Ӧѡ�õ��Լ�Ϊ ��
��2��װ��F�������� ��װ��G�������� ��
��3������װ��G����F��ClNO���ܷ�����Ӧ�Ļ�ѧ����ʽΪ ��
��4��ijͬѧ��Ϊ����������Һֻ������������ClNO������������NO������װ��H������Ч��ȥ�ж����塣Ϊ�����һ���⣬�ɽ�β����ij������ͬʱͨ������������Һ�У���������Ļ�ѧʽ�� ��
| ����ʽ | ���� | �۵� | �е� | �ܽ��� | ��״ |
| ClNO | �Ȼ������� | -64.5�� | -5.5�� | ����Ũ���� | ���ɫҺ����ж����壬���д̼��������ˮ��Ӧ���ɵ������������Ȼ��� |

�Իش��������⣺
��1������D��װ�д�����NO���壬��B��Ӧѡ�õ��Լ�Ϊ ��ʵ��ʱ����B��ȥijЩ�������岢ͨ���۲�B�е��������жϷ�Ӧ���ʣ�B�����е������� ������D��װ�д�������������C��Ӧѡ�õ��Լ�Ϊ ��
��2��װ��F�������� ��װ��G�������� ��
��3������װ��G����F��ClNO���ܷ�����Ӧ�Ļ�ѧ����ʽΪ ��
��4��ijͬѧ��Ϊ����������Һֻ������������ClNO������������NO������װ��H������Ч��ȥ�ж����塣Ϊ�����һ���⣬�ɽ�β����ij������ͬʱͨ������������Һ�У���������Ļ�ѧʽ�� ��
��1���ٱ���ʳ��ˮ��2�֣���ȫƿ����ֹ��װ�ö��������¹ʣ���2�֣� ��Ũ���� ��2�֣�
��2�������ռ��������ȣ�2�֣� ��ֹˮ��������U�ܣ�2�֣�
��3��2ClNO+H2O =2HCl+NO��+NO2��(��2ClNO+H2O=2HCl+N2O3��) ��2�֣�
��4��O2��2�֣�
��2�������ռ��������ȣ�2�֣� ��ֹˮ��������U�ܣ�2�֣�
��3��2ClNO+H2O =2HCl+NO��+NO2��(��2ClNO+H2O=2HCl+N2O3��) ��2�֣�
��4��O2��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
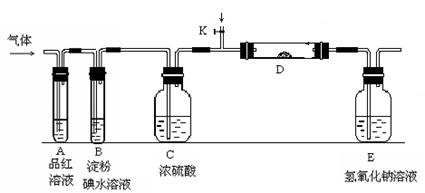
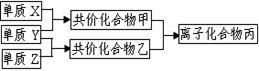
 ��3������Z��ȼ�տ��������ֲ������һ�ֲ��ﶡ�����и�ԭ������㲻ȫ��8���ӽṹ�����Ļ�ѧʽ��__________��
��3������Z��ȼ�տ��������ֲ������һ�ֲ��ﶡ�����и�ԭ������㲻ȫ��8���ӽṹ�����Ļ�ѧʽ��__________��
 �� �Ȼ���ϡ��Һ �� �Ȼ�������Һ �� ���軯����Һ
�� �Ȼ���ϡ��Һ �� �Ȼ�������Һ �� ���軯����Һ
 ______________________��
______________________�� ���ķ�����____________________________________________________��
���ķ�����____________________________________________________��