��Ŀ����
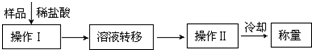
 ���к�CaO���ʵ�CaC2������ij�о���ѧϰС���ͬѧ�����������ַ����ⶨCaC2�����Ĵ��ȣ�����д���пհף�
���к�CaO���ʵ�CaC2������ij�о���ѧϰС���ͬѧ�����������ַ����ⶨCaC2�����Ĵ��ȣ�����д���пհף�
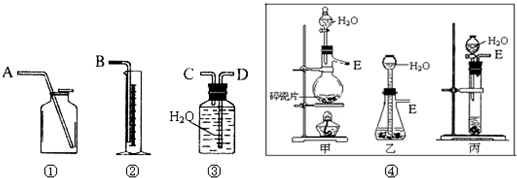
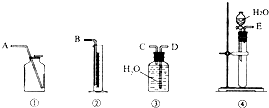
��1����һ�ַ����������ͼ��ѡ���ʵ���װ�ã����һ��ʵ�飬�ⶨCaC2�����Ĵ��ȣ���ѡ��װ�õ�����˳��Ӧ�ǣ�����ӿڵ���ĸ����______��
| �������� | ����/g | |
| ��ƿ+ˮ+���� | ��1�� | 196.30 |
| ��2�� | 196.15 | |
| ��3�� | 196.05 | |
| ��4�� | 196.00 | |
| ��5�� | 196.00 |
����CaC2����������ʱ�������õ�������______����������6�ζ�����ԭ����______����������CaC2����������Ϊ______��
��3�������ַ�������ȡһ��������������1.60g���������������£�

�ٲ������������______��
�ڻ���ֱ�Ӳⶨ����������______��
����ת����Һʱ������Һת�Ʋ���ȫ����CaC2���������IJⶨ���______���ƫ����ƫС�����䡱����
����ָ����ʵ�鷽����2������֮��______
����Ľ����ʵ�鷽�������ٽ������1������֮��______��
�⣺��1����Ȳ����������ˮ��������ˮ���������ⶨ���������װ�õ�����˳����E��C��D��B��
�ʴ�Ϊ��װ�õ�����˳����E��C��D��B��
��2������ƿ��������Ȳ�������ݳ���ʣ�����ʵ�������С�����Ը��ݳ�����Ӧǰ����ƿ�����ʵ������仯���õ�������������������ݻ�ѧ����ʽ������Ȳ���������õ���Ʒ�Ĵ��ȣ�������Ҫ������Ӧǰ��ƿ��ˮ����������Ʒ��������Ӧ����ƿ��ˮ��ʽ������������4��5��ʵ�������ͬ��������ƿ�������Ѵ���أ���Ӧ������ȫ��˵����Ӧ�ڵ��Ĵζ���ʱ�Ѿ�������
��ƿ�з�����ӦCaC2+2H2O��Ca��OH��2+C2H2��������C2H2������Ϊ��195+1.5��g-196g=0.5g����CaC2����Ϊm����
CaC2+2H2O��Ca��OH��2+C2H2��
64g 26g
m 0.5g
����m= =
=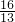 g��CaC2����������Ϊ
g��CaC2����������Ϊ ��100%=82%��
��100%=82%��
�ʴ�Ϊ��������������ƿ��ˮ����������4��5�ζ�������4��5��ʵ�������ͬ��������ƿ�������Ѵ���أ���Ӧ������ȫ��82%
��3���ټ������ᷴӦ�������Ȼ�����Һ��ʵ��Ŀ�����������Ȼ��Ƶ�������Ӧ����Һ��������������Ϊ�������ò��������裬���Ⱥ����ù�����ڸ���������ȴ�������
�ʴ�Ϊ��������
�ڼ������ᷴӦ�������Ȼ�����Һ��ʵ��Ŀ�����������Ȼ��Ƶ���������Ҫֱ�ӲⶨCaCl2������
�ʴ�Ϊ��CaCl2������
����ת����Һʱ������Һת�Ʋ���ȫ���ᵼ�²ⶨ���Ȼ��Ƶ�����ƫС����ԭ��Ʒ�е����������ʵ���ΪX��̼�������ʵ���ΪY��δ���ʱ�������Ȼ�������ΪZ����õ���56X+64Y=1.6��X+Y=Z/111��������Y=0.2- �����Գ������Ȼ������Խ�࣬����õ���̼���Ƶ�����Խ����ռ��������Խ����ת����Һʱ������Һת�Ʋ���ȫ������Ȼ��ƣ�Z���٣�Y����CaC2���������������Բ������ƫ��
�����Գ������Ȼ������Խ�࣬����õ���̼���Ƶ�����Խ����ռ��������Խ����ת����Һʱ������Һת�Ʋ���ȫ������Ȼ��ƣ�Z���٣�Y����CaC2���������������Բ������ƫ��
�ʴ�Ϊ��ƫ��
�ܸ�ʵ�鷽���IJ���֮��������CaCl2���к�ǿ����ˮ�ԣ������IJ�һ������ˮCaCl2��������CaO��CaC2��Ħ�������ӽ���ʵ������ܴ�
�ʴ�Ϊ������CaCl2���к�ǿ����ˮ�ԣ������IJ�һ������ˮCaCl2��������CaO��CaC2��Ħ�������ӽ���ʵ������ܴ�
������CaCl2���к�ǿ����ˮ�ԣ������IJ�һ������ˮCaCl2�����������Խ�Ca2+ת��ΪCaCO3��CaC2O4���������˺����������
�ʴ�Ϊ�����Խ�Ca2+ת��ΪCaCO3��CaC2O4���������˺����������
��������1����Ȳ����������ˮ��������ˮ���������ⶨ���������
��2������ƿ��������Ȳ�������ݳ���ʣ�����ʵ�������С�����Ը��ݳ�����Ӧǰ����ƿ�����ʵ������仯���õ�������������������ݻ�ѧ����ʽ������Ȳ���������õ���Ʒ�Ĵ��ȣ�������Ҫ������Ӧǰ��ƿ��ˮ����������Ʒ��������Ӧ����ƿ��ˮ��ʽ����������
��4��5��ʵ�������ͬ��������ƿ�������Ѵ���أ���Ӧ������ȫ��˵����Ӧ�ڵ��Ĵζ���ʱ�Ѿ�������
��ƿ�з�����ӦCaC2+2H2O��Ca��OH��2+C2H2������ƿ+ˮ+��������������Ϊ����C2H2�����������ݷ���ʽ������Ʒ��CaC2����������������CaC2������������
��3���ٸ��ݷ�Ӧ���̺����ɲ�������ʷ����жϣ�ͨ��������Һ�õ����壬��������������
�ڼ������ᷴӦ�������Ȼ�����Һ��ʵ��Ŀ�����������Ȼ��Ƶ�������Ӧ����Һ�����������ڸ���������ȴ���������������
����ת����Һʱ������Һת�Ʋ���ȫ���ᵼ�²ⶨ���Ȼ��Ƶ�����ƫС����ԭ��Ʒ�е����������ʵ���ΪX��̼�������ʵ���ΪY��δ���ʱ�������Ȼ�������ΪZ����õ���56X+64Y=1.6��X+Y=Z/111�������жϣ�
������CaCl2���к�ǿ����ˮ�ԣ������IJ�һ������ˮCaCl2��������CaO��CaC2��Ħ�������ӽ���ʵ������ܴ�
������CaCl2���к�ǿ����ˮ�ԣ������IJ�һ������ˮCaCl2����������Ca2+ת��ΪCaCO3��CaC2O4���������˺����������
���������⿼��ѧ���Ե�ʵ�鷽��ԭ�������������ۡ�������ɺ����IJⶨ����ѧ����ȣ���Ŀ�ѶȽϴ�����ʵ��ԭ���ǹؼ����Ƕ�ѧ���ۺ������뿼�飬��Ҫѧ���߱���ʵ�Ļ���֪ʶ��
�ʴ�Ϊ��װ�õ�����˳����E��C��D��B��
��2������ƿ��������Ȳ�������ݳ���ʣ�����ʵ�������С�����Ը��ݳ�����Ӧǰ����ƿ�����ʵ������仯���õ�������������������ݻ�ѧ����ʽ������Ȳ���������õ���Ʒ�Ĵ��ȣ�������Ҫ������Ӧǰ��ƿ��ˮ����������Ʒ��������Ӧ����ƿ��ˮ��ʽ������������4��5��ʵ�������ͬ��������ƿ�������Ѵ���أ���Ӧ������ȫ��˵����Ӧ�ڵ��Ĵζ���ʱ�Ѿ�������
��ƿ�з�����ӦCaC2+2H2O��Ca��OH��2+C2H2��������C2H2������Ϊ��195+1.5��g-196g=0.5g����CaC2����Ϊm����
CaC2+2H2O��Ca��OH��2+C2H2��
64g 26g
m 0.5g
����m=
 =
=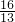 g��CaC2����������Ϊ
g��CaC2����������Ϊ ��100%=82%��
��100%=82%���ʴ�Ϊ��������������ƿ��ˮ����������4��5�ζ�������4��5��ʵ�������ͬ��������ƿ�������Ѵ���أ���Ӧ������ȫ��82%
��3���ټ������ᷴӦ�������Ȼ�����Һ��ʵ��Ŀ�����������Ȼ��Ƶ�������Ӧ����Һ��������������Ϊ�������ò��������裬���Ⱥ����ù�����ڸ���������ȴ�������
�ʴ�Ϊ��������
�ڼ������ᷴӦ�������Ȼ�����Һ��ʵ��Ŀ�����������Ȼ��Ƶ���������Ҫֱ�ӲⶨCaCl2������
�ʴ�Ϊ��CaCl2������
����ת����Һʱ������Һת�Ʋ���ȫ���ᵼ�²ⶨ���Ȼ��Ƶ�����ƫС����ԭ��Ʒ�е����������ʵ���ΪX��̼�������ʵ���ΪY��δ���ʱ�������Ȼ�������ΪZ����õ���56X+64Y=1.6��X+Y=Z/111��������Y=0.2-
 �����Գ������Ȼ������Խ�࣬����õ���̼���Ƶ�����Խ����ռ��������Խ����ת����Һʱ������Һת�Ʋ���ȫ������Ȼ��ƣ�Z���٣�Y����CaC2���������������Բ������ƫ��
�����Գ������Ȼ������Խ�࣬����õ���̼���Ƶ�����Խ����ռ��������Խ����ת����Һʱ������Һת�Ʋ���ȫ������Ȼ��ƣ�Z���٣�Y����CaC2���������������Բ������ƫ���ʴ�Ϊ��ƫ��
�ܸ�ʵ�鷽���IJ���֮��������CaCl2���к�ǿ����ˮ�ԣ������IJ�һ������ˮCaCl2��������CaO��CaC2��Ħ�������ӽ���ʵ������ܴ�
�ʴ�Ϊ������CaCl2���к�ǿ����ˮ�ԣ������IJ�һ������ˮCaCl2��������CaO��CaC2��Ħ�������ӽ���ʵ������ܴ�
������CaCl2���к�ǿ����ˮ�ԣ������IJ�һ������ˮCaCl2�����������Խ�Ca2+ת��ΪCaCO3��CaC2O4���������˺����������
�ʴ�Ϊ�����Խ�Ca2+ת��ΪCaCO3��CaC2O4���������˺����������
��������1����Ȳ����������ˮ��������ˮ���������ⶨ���������
��2������ƿ��������Ȳ�������ݳ���ʣ�����ʵ�������С�����Ը��ݳ�����Ӧǰ����ƿ�����ʵ������仯���õ�������������������ݻ�ѧ����ʽ������Ȳ���������õ���Ʒ�Ĵ��ȣ�������Ҫ������Ӧǰ��ƿ��ˮ����������Ʒ��������Ӧ����ƿ��ˮ��ʽ����������
��4��5��ʵ�������ͬ��������ƿ�������Ѵ���أ���Ӧ������ȫ��˵����Ӧ�ڵ��Ĵζ���ʱ�Ѿ�������
��ƿ�з�����ӦCaC2+2H2O��Ca��OH��2+C2H2������ƿ+ˮ+��������������Ϊ����C2H2�����������ݷ���ʽ������Ʒ��CaC2����������������CaC2������������
��3���ٸ��ݷ�Ӧ���̺����ɲ�������ʷ����жϣ�ͨ��������Һ�õ����壬��������������
�ڼ������ᷴӦ�������Ȼ�����Һ��ʵ��Ŀ�����������Ȼ��Ƶ�������Ӧ����Һ�����������ڸ���������ȴ���������������
����ת����Һʱ������Һת�Ʋ���ȫ���ᵼ�²ⶨ���Ȼ��Ƶ�����ƫС����ԭ��Ʒ�е����������ʵ���ΪX��̼�������ʵ���ΪY��δ���ʱ�������Ȼ�������ΪZ����õ���56X+64Y=1.6��X+Y=Z/111�������жϣ�
������CaCl2���к�ǿ����ˮ�ԣ������IJ�һ������ˮCaCl2��������CaO��CaC2��Ħ�������ӽ���ʵ������ܴ�
������CaCl2���к�ǿ����ˮ�ԣ������IJ�һ������ˮCaCl2����������Ca2+ת��ΪCaCO3��CaC2O4���������˺����������
���������⿼��ѧ���Ե�ʵ�鷽��ԭ�������������ۡ�������ɺ����IJⶨ����ѧ����ȣ���Ŀ�ѶȽϴ�����ʵ��ԭ���ǹؼ����Ƕ�ѧ���ۺ������뿼�飬��Ҫѧ���߱���ʵ�Ļ���֪ʶ��

��ϰ��ϵ�д�
�����Ŀ
 ���к�CaO���ʵ�CaC2������ij�о���ѧϰС���ͬѧ�����������ַ����ⶨCaC2�����Ĵ��ȣ�����д���пհף�
���к�CaO���ʵ�CaC2������ij�о���ѧϰС���ͬѧ�����������ַ����ⶨCaC2�����Ĵ��ȣ�����д���пհף�