��Ŀ����
��2013?����ģ�⣩ij�о���ѧϰС�齫һ��Ũ�ȵ�Na2CO3��Һ����CuSO4��Һ�еõ���ɫ������
��ͬѧ��Ϊ����������CuCO3��
��ͬѧ��Ϊ����������Cu��OH��2��
��ͬѧ��Ϊ����������CuCO3��Cu��OH��2�Ļ������߶������ᾧˮ����
������ͬѧ�Ĺ۵㣬����Ϊ��ԭ����
��������������ɫ����ϴ�ӡ����������Թ��м��ȣ�����̽��������ijɷ֣�
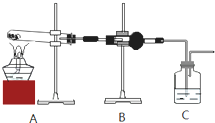
��1��Bװ�����Լ��Ļ�ѧʽ��
��2����֤����ͬѧ�۵���ȷ��ʵ��������
���о���ѧϰС���ͬѧΪ�˲ⶨһ����CuCO3��Cu��OH��2��ɵĻ������Cu��OH��2��������������Ƴ�����װ�ý���ʵ�飮
��1����װ�����ӵ�˳��Ϊ
��2��ʵ�鿪ʼ�ͽ���ʱ��Ҫͨ������Ŀ���������ʱͨ�����������������
��3����������Ʒ������Ϊm g��װ��D����������n g��������Cu��OH��2����������Ϊ
%
%��
��4����������װ�ò����Cu��OH��2��������ƫ�ͣ���ԭ����
��ͬѧ��Ϊ����������CuCO3��
��ͬѧ��Ϊ����������Cu��OH��2��
��ͬѧ��Ϊ����������CuCO3��Cu��OH��2�Ļ������߶������ᾧˮ����
������ͬѧ�Ĺ۵㣬����Ϊ��ԭ����
Cu2++CO32-+H2O=Cu��OH��2��+CO2��
Cu2++CO32-+H2O=Cu��OH��2��+CO2��
�������ӷ���ʽ��ʾ������������������ɫ����ϴ�ӡ����������Թ��м��ȣ�����̽��������ijɷ֣�

��1��Bװ�����Լ��Ļ�ѧʽ��
CuSO4
CuSO4
��Cװ�����Լ�������������ʯ��ˮ
����ʯ��ˮ
����2����֤����ͬѧ�۵���ȷ��ʵ��������
B�а�ɫ�����Ϊ��ɫ��C�г���ʯ��ˮ�������
B�а�ɫ�����Ϊ��ɫ��C�г���ʯ��ˮ�������
�����о���ѧϰС���ͬѧΪ�˲ⶨһ����CuCO3��Cu��OH��2��ɵĻ������Cu��OH��2��������������Ƴ�����װ�ý���ʵ�飮

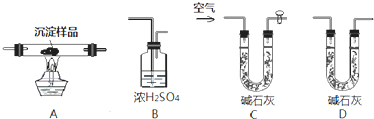
��1����װ�����ӵ�˳��Ϊ
CABD
CABD
����2��ʵ�鿪ʼ�ͽ���ʱ��Ҫͨ������Ŀ���������ʱͨ�����������������
���Խ�װ����������ˮ�����Ͷ�����̼�ϳ�
���Խ�װ����������ˮ�����Ͷ�����̼�ϳ�
����3����������Ʒ������Ϊm g��װ��D����������n g��������Cu��OH��2����������Ϊ
| 100(11m-31n) |
| 11m |
| 100(11m-31n) |
| 11m |
��4����������װ�ò����Cu��OH��2��������ƫ�ͣ���ԭ����
�����е�ˮ�����Ͷ�����̼��װ��D�еļ�ʯ������
�����е�ˮ�����Ͷ�����̼��װ��D�еļ�ʯ������
����������CO32-ˮ��ɼ��ԣ�Cu2+��OH?�������Cu��OH��2��
��1�����ü��ȵķ������飬������ͭ��̼��ͭ���ȷֽ�õ�����ͭ��ˮ�Ͷ�����̼������������ͭ������ˮ����ͭ���飬����̼��ͭ���ó����ʯ��ˮ��������Ķ�����̼������ʯ��ˮ�����˵������CuCO3��
��2����������Cu��OH��2������������ͭ�õ�����ͭ��ˮ��B����ˮ����ͭ������C�г���ʯ��ˮ������ǣ�
��1������CuCO3��Cu��OH��2���ȷֽ�IJ���ͼ���������������������װ�ã�
��2����ʯ�ҿ�������ˮ�Ͷ�����̼�������е�ˮ�Լ�������̼�����������Ӧ�ý����ų�����
��3��װ��D����������n�ˣ�������ˮ��������ng������ԭ���غ����õ���
��4������װ�ò����Cu��OH��2��������ƫ�ͣ�����Ϊ����ʯ�����ն�����̼��ͬʱ���տ����еĶ�����̼��ˮ������ʹ̼��ͭ��������������ͭ����������С��
��1�����ü��ȵķ������飬������ͭ��̼��ͭ���ȷֽ�õ�����ͭ��ˮ�Ͷ�����̼������������ͭ������ˮ����ͭ���飬����̼��ͭ���ó����ʯ��ˮ��������Ķ�����̼������ʯ��ˮ�����˵������CuCO3��
��2����������Cu��OH��2������������ͭ�õ�����ͭ��ˮ��B����ˮ����ͭ������C�г���ʯ��ˮ������ǣ�
��1������CuCO3��Cu��OH��2���ȷֽ�IJ���ͼ���������������������װ�ã�
��2����ʯ�ҿ�������ˮ�Ͷ�����̼�������е�ˮ�Լ�������̼�����������Ӧ�ý����ų�����
��3��װ��D����������n�ˣ�������ˮ��������ng������ԭ���غ����õ���
��4������װ�ò����Cu��OH��2��������ƫ�ͣ�����Ϊ����ʯ�����ն�����̼��ͬʱ���տ����еĶ�����̼��ˮ������ʹ̼��ͭ��������������ͭ����������С��
����⣺����ͬѧ��Ϊ����������Cu��OH��2��ԭ����CO32-ˮ��ɼ��ԣ�Cu2+��OH?�������Cu��OH��2����Ӧ�����ӷ���ʽΪCu2++CO32-+H2O=Cu��OH��2��+CO2����
�ʴ�Ϊ��Cu2++CO32-+H2O=Cu��OH��2��+CO2����
��1��װ��B�����Ƿ���ˮ���ɣ�������ˮ����ͭ���飬����ˮ����ͭ����ɫ˵����ˮ���ɣ���֤��������������ͭ���ɣ������������������ͭ��
�ó����ʯ��ˮ�����Ƿ����������̼��װ��C�г���ʯ��ˮ����ǣ�˵�����ɶ�����̼����˵������CuCO3��
�ʴ�Ϊ��CuSO4������ʯ��ˮ��
��2����������Cu��OH��2������������ͭ�õ�����ͭ��ˮ��B����ˮ����ͭ������C����ʯ��ˮ������ǣ�
�ʴ�Ϊ��B����ˮ����ͭ������C����ʯ��ˮ������ǣ�
��1����ʯ�ҿ�������ˮ�Ͷ�����̼�������е�ˮ�Լ�������̼�����������Ӧ�ý�����ʵ��֮ǰ�ų���������װ�õ������ϼ�ʯ�ң���ֹ�����е�ˮ�Ͷ�����̼��������������������Ũ����������ˮ���ü�ʯ�������ն�����̼������Ũ����ͼ�ʯ�ҵ���������ȷ������ˮ�Ͷ�����̼�������ʴ�Ϊ��CABD��
��2��װ��C�м�ʯ�ҵ������ǣ����տ����е�H2O ������CO2��ʵ�鿪ʼʱ��ʵ�����ʱ��Ҫͨ������Ҵ������Ŀ��������ö��ǽ�װ����������H2O ������CO2�ų���
�ʴ�Ϊ����װ����������ˮ������CO2�ų���
��3����������Ʒ������Ϊm g��װ��D����������n g������װ��ͼ������֪��D����������Ϊ������̼������������ԭ���غ����̼�������=
��124g/mol��
������ͭ����=mg-
g��
����������ͭ��������=
��100%=
%���ʴ�Ϊ��
%��
��4������ʯ�����ն�����̼��ͬʱ���տ����еĶ�����̼��ˮ������ʹ̼��ͭ��������������ͭ����������С��
�ʴ�Ϊ�������е�ˮ�����Ͷ�����̼��װ��D�еļ�ʯ�����գ�
�ʴ�Ϊ��Cu2++CO32-+H2O=Cu��OH��2��+CO2����
��1��װ��B�����Ƿ���ˮ���ɣ�������ˮ����ͭ���飬����ˮ����ͭ����ɫ˵����ˮ���ɣ���֤��������������ͭ���ɣ������������������ͭ��
�ó����ʯ��ˮ�����Ƿ����������̼��װ��C�г���ʯ��ˮ����ǣ�˵�����ɶ�����̼����˵������CuCO3��
�ʴ�Ϊ��CuSO4������ʯ��ˮ��
��2����������Cu��OH��2������������ͭ�õ�����ͭ��ˮ��B����ˮ����ͭ������C����ʯ��ˮ������ǣ�
�ʴ�Ϊ��B����ˮ����ͭ������C����ʯ��ˮ������ǣ�
��1����ʯ�ҿ�������ˮ�Ͷ�����̼�������е�ˮ�Լ�������̼�����������Ӧ�ý�����ʵ��֮ǰ�ų���������װ�õ������ϼ�ʯ�ң���ֹ�����е�ˮ�Ͷ�����̼��������������������Ũ����������ˮ���ü�ʯ�������ն�����̼������Ũ����ͼ�ʯ�ҵ���������ȷ������ˮ�Ͷ�����̼�������ʴ�Ϊ��CABD��
��2��װ��C�м�ʯ�ҵ������ǣ����տ����е�H2O ������CO2��ʵ�鿪ʼʱ��ʵ�����ʱ��Ҫͨ������Ҵ������Ŀ��������ö��ǽ�װ����������H2O ������CO2�ų���
�ʴ�Ϊ����װ����������ˮ������CO2�ų���
��3����������Ʒ������Ϊm g��װ��D����������n g������װ��ͼ������֪��D����������Ϊ������̼������������ԭ���غ����̼�������=
| ng |
| 44g/mol |
������ͭ����=mg-
| 124n |
| 44 |
����������ͭ��������=
mg-
| ||
| mg |
| 100(11m-31n) |
| 11m |
| 100(11m-31n) |
| 11m |
��4������ʯ�����ն�����̼��ͬʱ���տ����еĶ�����̼��ˮ������ʹ̼��ͭ��������������ͭ����������С��
�ʴ�Ϊ�������е�ˮ�����Ͷ�����̼��װ��D�еļ�ʯ�����գ�
���������⿼�����������ʵ�ʵ����֤���������̽�������ʺ����IJⶨ�����ͼ��㣬װ�õ�ѡ��ԭ���������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 ��2013?����ģ�⣩���ޣ�Salol����һ����������Ϊ�����廯��������ģ������ͼ��ʾ��ͼ��������֮������ߴ�����ѧ�����絥����˫���ȣ���������˵������ȷ���ǣ�������
��2013?����ģ�⣩���ޣ�Salol����һ����������Ϊ�����廯��������ģ������ͼ��ʾ��ͼ��������֮������ߴ�����ѧ�����絥����˫���ȣ���������˵������ȷ���ǣ������� ��������
��������