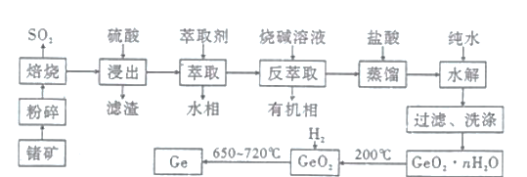
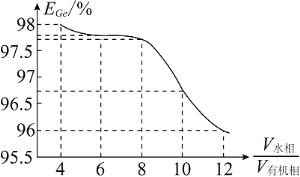
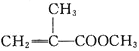��Ŀ����
����Ŀ�����ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�ã���![]() ֱ���ŷŻ�Ի������Σ����
ֱ���ŷŻ�Ի������Σ����
I.��֪���ؾ�ʯ![]() ���¶��տɷ���һϵ�з�Ӧ�����в��ַ�Ӧ���£�
���¶��տɷ���һϵ�з�Ӧ�����в��ַ�Ӧ���£�
![]()
![]()
![]()
![]()
��֪��![]()
![]()
��![]()
![]() _____________��
_____________��
![]() ��β������ͨ�������¼��ַ�����
��β������ͨ�������¼��ַ�����
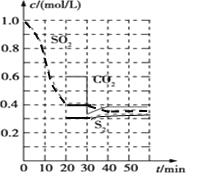
(1)����̿��ԭ�� ��Ӧԭ�������º���ʱ![]() ��Ӧ���е���ͬʱ���ø����ʵ�Ũ������ͼ��
��Ӧ���е���ͬʱ���ø����ʵ�Ũ������ͼ��
![]() ��Ӧ���ʱ�ʾΪ
��Ӧ���ʱ�ʾΪ![]() _________________��
_________________��
![]() ʱ���ı�ijһ����ƽ�ⷢ���ƶ�����ı���������п�����________________________��
ʱ���ı�ijһ����ƽ�ⷢ���ƶ�����ı���������п�����________________________��
![]() ʱ��ƽ�ⳣ��
ʱ��ƽ�ⳣ��![]() _____________��
_____________��
(2)�����������շ�
![]() ��Һ����
��Һ����![]() �����ӷ���ʽΪ____________
�����ӷ���ʽΪ____________
![]() �����£���������
�����£���������![]() ʱ������Һ���������Ũ�ȹ�ϵһ����ȷ����_______
ʱ������Һ���������Ũ�ȹ�ϵһ����ȷ����_______![]() �����
�����![]()
![]()
![]()
![]()
![]() ˮ�����
ˮ�����![]()
(3)�绯ѧ������
![]() ����ͼ��ʾ��
����ͼ��ʾ��![]() ��
��![]() �缫�ķ�ӦʽΪ___________________��
�缫�ķ�ӦʽΪ___________________��
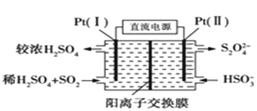
![]() ����·��ת��
����·��ת��![]() ʱ
ʱ![]() ��Ũ
��Ũ![]() ��δ�ų�
��δ�ų�![]() ������Ĥ�����Һ������_______mol���ӣ�
������Ĥ�����Һ������_______mol���ӣ�
���𰸡�![]()
![]() ��С
��С![]() ��Ũ��
��Ũ�� ![]()
![]()
![]()
![]()
![]()
��������
����֪��BaSO4(s)+4C(s)=BaS(s)+4CO(g)��H=+571.2kJmol-1��
��BaS(s)=Ba(s)+S(s)��H=+460kJmol-1��
��2C(s)+O2(g)=2CO(g)��H=-221kJmol-1��
���ݸ�˹���ɣ�����2-��-�ڵ÷���ʽBa(s)+S(s)+2O2(g)=BaSO4��s���ݴ˼��㣻
��1���ٸ���v=![]() ����v(SO2)��
����v(SO2)��
��30minʱ˲�䣬������̼Ũ�Ƚ��ͣ�S2��Ũ�Ȳ��䣬���������̼��S2��Ũ�Ⱦ�����Ӧ�Ǽ���CO2��Ũ�ȣ�
��ƽ�ⳣ��K=![]() ��ע�����ʹ�Һ�岻д�����ʽ��
��ע�����ʹ�Һ�岻д�����ʽ��
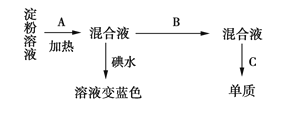
��2����Na2SO3��Һ��SO2��Ӧ�������������ƣ�
��a�����ݵ���غ��жϣ�
b�����������غ��жϣ�
c��NaHSO3��Һ��HSO3-�ĵ���̶ȴ�����ˮ��̶ȣ�
d��ˮ�����������Ũ�ȵ�����Һ������������Ũ�ȣ�����NaHSO3��Һ��pHδ֪�����ܼ���ˮ�����������Ũ�ȣ�
��3������ͼ��֪��Pt��1���缫�϶������������������
�����缫��ӦʽΪ��SO2-2e-+2H2O=SO42-+4 H+�����ݵ���ת���غ��������������������ӵ����ʵ�����Ϊ������Һ�����ԣ������������ͨ�������ӽ���Ĥ�����Ҳ࣬�����Һ����������Ϊ����������������������
��.��֪��BaSO4(s)+4C(s)=BaS(s)+4CO(g)��H=+571.2kJmol-1
��BaS(s)=Ba(s)+S(s)��H=+460kJmol-1
��2C(s)+O2(g)=2CO(g)��H=-221kJmol-1��
���ݸ�˹���ɣ�����2���ڵ÷���ʽBa(s)+S(s)+2O2(g)=BaSO4(s)��H=(-221)��2-(+460)-(+571.2)=-1473.2kJmol-1��
�ʴ�Ϊ��-1473.2kJmol-1��
��.(1)����ͼ��֪��0~20min�ڶ�������Ũ�ȱ仯��Ϊ1mol/L-0.4mol/L=0.6mol/L����v(SO2)=![]() =0.03mol/(Lmin)��
=0.03mol/(Lmin)��
�ʴ�Ϊ��0.03mol/(Lmin)��
�� 30minʱ˲�䣬������̼Ũ�Ƚ��ͣ�S2��Ũ�Ȳ��䣬���������̼��S2��Ũ�Ⱦ�����Ӧ�Ǽ���CO2��Ũ�ȣ�
�ʴ�Ϊ������CO2��Ũ�ȣ�
![]() ��Ϊ�¶�û�иı䣬40minʱ��ƽ�ⳣ����20minʱ��ȣ���20minʱ��ƽ��״̬����ƽ�ⳣ����
��Ϊ�¶�û�иı䣬40minʱ��ƽ�ⳣ����20minʱ��ȣ���20minʱ��ƽ��״̬����ƽ�ⳣ����

![]() ����K
����K![]() ��
��
�ʴ�Ϊ��0.675��
(2)��Na2SO3��Һ��SO2��Ӧ�������������ƣ���Ӧ���ӷ���ʽΪ��SO32+SO2+H2O=2HSO3��
�ʴ�Ϊ��SO32+SO2+H2O=2HSO3��
��a.���ݵ���غ㣺c(Na+)+c(H+)=2c(SO32)+c(HSO3)+c(OH)������Һ��c(Na+)+c(H+)>c(SO32)+c(HSO3)+c(OH)����a��ȷ��
b.��Һ��SԪ����SO32��HSO3��H2SO3��ʽ���ڣ�NaԪ������Ԫ�����ʵ���֮��Ϊ1:1������Һ��c(Na+)=c(SO32)+c(HSO3)+c(H2SO4)����b��ȷ��
c.NaHSO3��Һ��HSO3�ĵ���̶ȴ�����ˮ��̶ȣ�����Һ��c(Na+)>c(HSO3)>c(H+)>c(SO32)> c(H2SO3)����c����
d.ˮ�����������Ũ�ȵ�����Һ������������Ũ�ȣ�����NaHSO3��Һ��pHδ֪�����ܼ���ˮ�����������Ũ�ȣ���d����
�ʴ�Ϊ��ab��
(3)����ͼ��֪��Pt(1)�缫�϶������������������ᣬ�缫��ӦʽΪ��SO22e+2H2O=SO42+4H+��
�ʴ�Ϊ��SO22e+2H2O=SO42+4H+��
�����缫��ӦʽΪ��SO22e+2H2O=SO42+4H+�����ݵ���ת���غ㣬������������ʵ���![]() =0.01mol������������Ϊ0.04mol��Ϊ������Һ�����ԣ�0.01mol�������Ҫ0.02mol�����ӣ������������ͨ�������ӽ���Ĥ�����Ҳ࣬����0.02mol�����������Ҳ࣬�������Һ����������Ϊ0.01mol+0.02mol=0.03mol��
=0.01mol������������Ϊ0.04mol��Ϊ������Һ�����ԣ�0.01mol�������Ҫ0.02mol�����ӣ������������ͨ�������ӽ���Ĥ�����Ҳ࣬����0.02mol�����������Ҳ࣬�������Һ����������Ϊ0.01mol+0.02mol=0.03mol��
�ʴ�Ϊ��0.03��

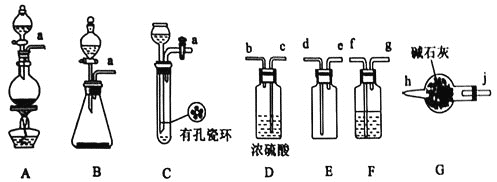
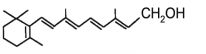
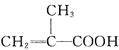
����Ŀ������ϩ������Ľṹ��ʽΪ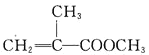 �����л������ĵ��壬��һ��ʵ�����Ʊ��������£�ʵ��װ����ͼ1��ʾ���г�װ�ü�������װ������ȥ����
�����л������ĵ��壬��һ��ʵ�����Ʊ��������£�ʵ��װ����ͼ1��ʾ���г�װ�ü�������װ������ȥ����
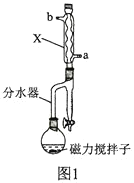
ʵ�鲽�����£�
��![]() ��ȡ86ml����ϩ��
��ȡ86ml����ϩ��![]()
![]() �����ձ��У��ڽ����ͬʱ����5mlŨ���ᣬ��ȴ�����£��ټ���50ml�״������裬��Ͼ��ȣ�
�����ձ��У��ڽ����ͬʱ����5mlŨ���ᣬ��ȴ�����£��ټ���50ml�״������裬��Ͼ��ȣ�
��![]() �������Һע��ͼ1װ�õķ�Ӧ���У�������������ӣ��������¶�Ϊ105�棬�������ȣ���ַ�Ӧ��
�������Һע��ͼ1װ�õķ�Ӧ���У�������������ӣ��������¶�Ϊ105�棬�������ȣ���ַ�Ӧ��
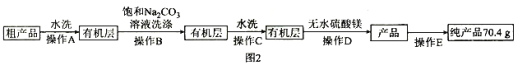
��![]() ������Ʒ��������ͼ2��ʾ��
������Ʒ��������ͼ2��ʾ��
��֪��
|
|
| |
�ܽ��� | �������л��ˮ | ��������ˮ���� | ������ˮ���������л��� |
�ܶ�/gcm-3 | 0.79 | 1.01 | 0.94 |
�е�/�� | 64.7 | 161 | 100~101 |
��Է������� | 32 | 86 | 100 |
�ش��������⣺
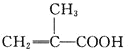
��1���Ʊ�����ϩ������Ļ�ѧ����ʽΪ______________________________��
��2��ͼ1������X������Ϊ________________�����ˮ��ӦΪ________________(����a������b��)�ڡ�
��3�����������ȿ�ȷ���Ʒ�Ӧ�¶Ⱥ�ʱ�䣬����Ӧ�¶ȿ��Ʋ��ã������и����������д��һ���л�������Ľṹ��ʽ��_________��
��4���ӷ�ˮ���м�ʱ�����ˮ��Ŀ����_____________________�������ˮ���е�ˮ�㲻�����������__________________________��
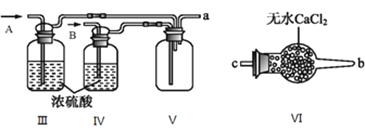
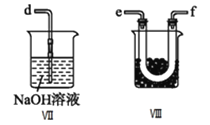
��5�����������У���������̼������Һϴ������Ŀ����_______________________________����ɲ���CӦѡ____________(��ѡ����ĸ����ͬ)װ�ã���ɲ���DӦѡ____________װ�á�
��6����ʵ���м���ϩ������IJ���Ϊ_________________![]() ������λ��Ч����
������λ��Ч����![]() ��
��