��Ŀ����
��ͭ��(CuFeS2)����ȡͭ���仯�������Ҫԭ��֮һ���������Ʊ������Ļ����ұ��ͭ�ķ�ӦΪ8CuFeS2��21O2![]() 8Cu��4FeO��2Fe2O3��16SO2��
8Cu��4FeO��2Fe2O3��16SO2��
(1)����ұ�����̲�������SO2�����д��������к�������_________(�����)��
a���߿��ŷ� b�������Ʊ�����
c���ô�����Һ������Na2SO4 d����Ũ��������
(2)���������(K2S2O8)����ǿ�����ԣ��ɽ�I-����ΪI2��![]() ��2I-====
��2I-====![]() ��I2��ͨ���ı䷴Ӧ;����Fe3+��Fe2+���ɴ�������Ӧ���������ӷ���ʽ��ʾFe3+��������Ӧ���Ĺ���:_____________��____________��(������ƽ)
��I2��ͨ���ı䷴Ӧ;����Fe3+��Fe2+���ɴ�������Ӧ���������ӷ���ʽ��ʾFe3+��������Ӧ���Ĺ���:_____________��____________��(������ƽ)
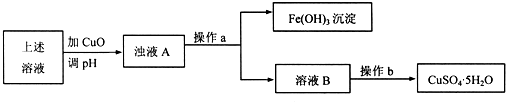
(3)���û�ͭ��ұ��ͭ������¯��(��Fe2O3��FeO��SiO2��Al2O3)���Ʊ�Fe2O3������Ϊ:
����ϡ�����ȡ¯�������ˡ�
����Һ���������ټ������NaOH��Һ�����ˣ�������ϴ�ӡ�������յ�Fe2O3��
a����ȥAl3+�����ӷ���ʽ��____________________________________��
b��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO��
�ṩ���Լ���ϡ���� ϡ���� KSCN��Һ KMnO4��Һ NaOH��Һ ��ˮ
��ѡ�Լ�Ϊ____________________________________________________________��
֤��¯���к���FeO��ʵ������Ϊ________________________________________��
(1)bc
(2)2Fe3+��2I-====2Fe2+��I2 ![]() ��2Fe2+====
��2Fe2+====![]() ��2Fe3+
��2Fe3+
(3)a��2Al3+��4OH-====![]() ��2H2O
��2H2O
b��ϡ���ᡢKMnO4��Һ ϡ�����ȡ¯��������ҺʹKMnO4��Һ��ɫ
����:
(1)SO2���ж����壬����ֱ���ſգ������ռ��Ʊ�������ô�����Һ������Na2SO4��
(2)2Fe3+��2I-====2Fe2+��I2��![]() ��2Fe2+====
��2Fe2+====![]() ��2Fe3+��
��2Fe3+��
(3)��ϡ�����ȡ¯����ʹFe2+��Fe3+��Al3+������Һ�ٹ��˳�SiO2��������Fe2+ʹ֮ת��ΪFe3+���ټ������NaOH��Һ������ӦAl3+��4OH-====![]() ��2H2O,�õ�����Fe(OH)3���ó�����ϴ�ӡ�������տɵ�Fe2O3��¯�����������FeO����ϡ�����ȡת��ΪFe2+��Fe2+���л�ԭ�ԣ�ʹKMnO4��Һ��ɫ��
��2H2O,�õ�����Fe(OH)3���ó�����ϴ�ӡ�������տɵ�Fe2O3��¯�����������FeO����ϡ�����ȡת��ΪFe2+��Fe2+���л�ԭ�ԣ�ʹKMnO4��Һ��ɫ��

 ��У����ϵ�д�
��У����ϵ�д�������Ļ������ڹ�ҵ������Ӧ�ù㷺���ش��������⣺
��1����ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ�����Ҫ�ɷ�ΪCuFeS2��
�ٲ��ij��ͭ��(CuFeS2)�к���20%����������������ÿ�ʯ��ͭ������������
������һ����Ȼ��ͭ��������ʯ����Ϊ�˲ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺��ȡ
��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ����н������գ�����Cu��Fe3O4��SO2���壬��100 mL���е��۵�
����ˮȫ������SO2��Ȼ��ȡ10mL����Һ����0.05mol/L������Һ���еζ�����ȥ������Һ����
��Ϊ20.00mL����û�ͭ��Ĵ��ȡ�
��2����FeS��Fe2O3�Ļ����56.6 g��������ϡH2SO4�ܽ��ɵ�3.2 g��ԭ�������FeS��������
��3��һ���¶��£�����ͭ���ȷֽ�����CuO��SO2��SO3��O2����֪��SO2��SO3���ܱ���ʯ�Һ�����
������Һ���ա�������ͼװ�ü�����ˮ����ͭ��ĩֱ����ȫ�ֽ⡣����ˮ����ͭ��ĩ����Ϊ10.0 g��
��ȫ�ֽ��װ�õ������仯��ϵ���±���ʾ��
|
װ�� |
A���Թ�+��ĩ�� |
B |
C |
|
��Ӧǰ |
42.0 g |
75.0 g |
140.0 g |
|
��Ӧ�� |
37.0 g |
79.0 g |
140.5 g |

��ͨ�����㣬�ƶϳ���ʵ������������ͭ�ֽ�Ļ�ѧ����ʽ��
��4������������Ƥ�����Ҫ��ѧ�Լ���������ˮNa2SO4��̿���ڸ����·�Ӧ�Ƶã���ѧ����ʽ���£�
��Na2SO4
+ 4C Na2S + 4CO�� ��Na2SO4
+ 4CO
Na2S + 4CO�� ��Na2SO4
+ 4CO Na2S + 4CO2
Na2S + 4CO2
a.���ڷ�Ӧ�����У�����CO��CO2�������Ϊ2mol��������Na2S�����ʵ�����
b.���ƾ�������ڿ����У��Ỻ��������Na2SO3��������Na2SO4���ֽ�43.72g���ֱ��ʵ�������Ʒ����ˮ�У���������������˵�4.8g������1.12L H2S ���壨��״����������Һ������ȫ���ݳ���������Һ�м���������BaCl2����˵�2.33g������������������Ʒ�ijɷּ������ʵ�����
 Cu2S+3SO2+2FeO
Cu2S+3SO2+2FeO 2Cu2O+2SO2
2Cu2O+2SO2