��Ŀ����
ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O)���������£�
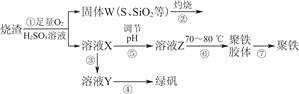
(1)�����̢��в���������ͨ��������Һ�У���Һ����ɫ����________��
A��Ʒ����Һ������ B����ɫʯ����Һ
C������KMnO4��Һ������ D����ˮ
(2)���̢��У�FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ______________________________
(3)���̢��У�������������________��
(4)���̢��У������ᾧ��Ҫʹ�þƾ��ơ����żܡ������ǣ�����Ҫ��������________________��
(5)���̢ݵ���pH��ѡ�������Լ��е�________(��ѡ�����)��
A��ϡ���ᡡ��B��CaCO3����C��NaOH��Һ
(6)���̢��У�����ҺZ���ȵ�70��80 �棬Ŀ����______________________��

(1)�����̢��в���������ͨ��������Һ�У���Һ����ɫ����________��
A��Ʒ����Һ������ B����ɫʯ����Һ
C������KMnO4��Һ������ D����ˮ
(2)���̢��У�FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ______________________________
(3)���̢��У�������������________��
(4)���̢��У������ᾧ��Ҫʹ�þƾ��ơ����żܡ������ǣ�����Ҫ��������________________��
(5)���̢ݵ���pH��ѡ�������Լ��е�________(��ѡ�����)��
A��ϡ���ᡡ��B��CaCO3����C��NaOH��Һ
(6)���̢��У�����ҺZ���ȵ�70��80 �棬Ŀ����______________________��
(1)ACD
(2)4FeS��3O2��6H2SO4=2Fe2(SO4)3��6H2O��4S
(3)Fe(����)��(4)������������(5)B
(6)�ٽ�Fe3����ˮ��
(2)4FeS��3O2��6H2SO4=2Fe2(SO4)3��6H2O��4S
(3)Fe(����)��(4)������������(5)B
(6)�ٽ�Fe3����ˮ��
(1)���̢��в�����������SO2������Ư����(��ʹƷ����Һ��ɫ)��SO2�����ָʾ��û��Ư�����á�
(2)FeS���ϼ�����Ϊ1��2��3, O2 ���ϼ۽���4�����߸�������4��3���ٽ��ԭ���غ���ƽ��
(3)���̢��м�������Fe3����ԭΪFe2�����ֲ��������ʡ�
(4)������Һʱ��Һ������������У��������ò��������衣
(5)�к������ᣬѡ��CaCO3���壬��ѡ��NaOH ��Һ������ʹFe3��������
(6)�����¶ȣ��ٽ�Fe3����ˮ�⡣
(2)FeS���ϼ�����Ϊ1��2��3, O2 ���ϼ۽���4�����߸�������4��3���ٽ��ԭ���غ���ƽ��
(3)���̢��м�������Fe3����ԭΪFe2�����ֲ��������ʡ�
(4)������Һʱ��Һ������������У��������ò��������衣
(5)�к������ᣬѡ��CaCO3���壬��ѡ��NaOH ��Һ������ʹFe3��������
(6)�����¶ȣ��ٽ�Fe3����ˮ�⡣

��ϰ��ϵ�д�
�����Ŀ

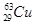 ����������������֮��Ϊ34
����������������֮��Ϊ34