��Ŀ����
����Ŀ�������㶹��Ϊ����Ѫҩ���ٴ�����Ҫ����Ԥ��������Ѫ����Ѫ˨�Լ�����ͨ�����·����ϳ�(���ַ�Ӧ����ʡ��)�����㶹�ء�
��֪�� +H2O
+H2O
��1��E��G��Ӧ��������_______��D�Ļ�ѧ����Ϊ_______�������㶹���к��еĹ���������Ϊ_______����2��C��D�Ļ�ѧ����ʽΪ_____________________________��
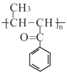
��3��д��G�Ľṹ��ʽ________________��
��4�����ӽṹ��ֻ����һ��������ͬʱ��������������G��ͬ���칹�干��_______����
�������Ȼ�����Һ������ɫ��Ӧ
������̼��������Һ��Ӧ���ɶ�����̼����
���У������ϵ�һ�ȴ���ֻ�����ֵ�ͬ���칹��Ľṹ��ʽΪ_______��
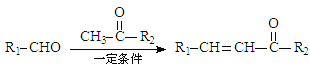
��5�����������ϳ�·�ߣ������CH3CH3��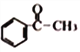 �ϳ�
�ϳ�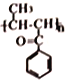 ��·������ͼ(���Լ���ѡ)��____________
��·������ͼ(���Լ���ѡ)��____________
���𰸡� �ӳɷ�Ӧ 4-��������ȩ(����������)ȩ �������ʻ�(ͪ��)������ 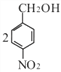 +O2
+O2![]()
 +2H2O
+2H2O 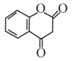 13
13 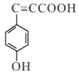 CH3CH3
CH3CH3![]() CH3CH2Cl
CH3CH2Cl![]() CH3CH2OH
CH3CH2OH![]() CH3CHO
CH3CHO
![]()
![]()

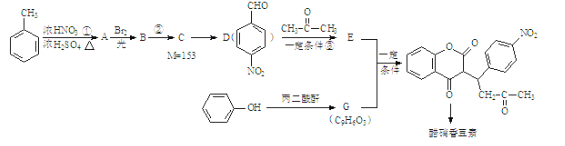
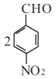
����������������� ![]() ��Ũ���ᡢŨ���������·�����������A�����D�Ľṹ��ʽ����֪A��
��Ũ���ᡢŨ���������·�����������A�����D�Ľṹ��ʽ����֪A�� ��Cһ������������
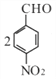
��Cһ������������![]() ������C����Է���������153����֪C��
������C����Է���������153����֪C��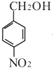 ��
��  �����ڹ�������������B����֪B��
�����ڹ�������������B����֪B��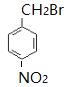 ��
��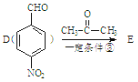 ������
������ +H2O����֪E��
+H2O����֪E�� �����ݴ����㶹�صĽṹ��ʽ������G��
�����ݴ����㶹�صĽṹ��ʽ������G�� ��
��
�������������Ϸ���, ��1�� ��
�� ��Ӧ����
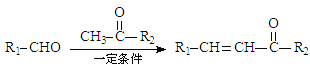
��Ӧ����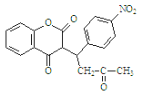 �������Ǽӳɷ�Ӧ��
�������Ǽӳɷ�Ӧ��![]() �Ļ�ѧ����Ϊ����������ȩ��
�Ļ�ѧ����Ϊ����������ȩ�� �к��еĹ���������Ϊ�������ʻ�����������2��
�к��еĹ���������Ϊ�������ʻ�����������2�� ��ͭ������������Ϊ
��ͭ������������Ϊ![]() ����ѧ����ʽΪ
����ѧ����ʽΪ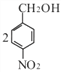 +O2
+O2![]()
 +2H2O��
+2H2O��
��3��G�Ľṹ��ʽ ����4�������Ȼ�����Һ������ɫ��Ӧ��˵�����з��ǻ���
����4�������Ȼ�����Һ������ɫ��Ӧ��˵�����з��ǻ���
����̼��������Һ��Ӧ���ɶ�����̼������˵�������Ȼ������ݲ����Ͷȣ���������һ��̼̼��������������2������![]() �����ڡ��䡢��3�ֽṹ ����������3������
�����ڡ��䡢��3�ֽṹ ����������3������![]() ����10�ֽṹ�����Է���������G��ͬ���칹�干13���������ϵ�һ�ȴ���ֻ��������˵��������������λȡ�������ṹ��ʽΪ
����10�ֽṹ�����Է���������G��ͬ���칹�干13���������ϵ�һ�ȴ���ֻ��������˵��������������λȡ�������ṹ��ʽΪ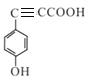 ����5��
����5�� Ҫ�Ʊ�
Ҫ�Ʊ�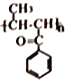 ��������������������ȡ����Ӧ���������飬������ˮ��Ϊ�Ҵ����Ҵ�������Ϊ��ȩ����ȩ��
��������������������ȡ����Ӧ���������飬������ˮ��Ϊ�Ҵ����Ҵ�������Ϊ��ȩ����ȩ�� һ������������
һ������������ ��
�� һ�������·����Ӿ۷�Ӧ����
һ�������·����Ӿ۷�Ӧ���� ���ϳ�·��ΪCH3CH3
���ϳ�·��ΪCH3CH3![]() CH3CH2Cl
CH3CH2Cl![]() CH3CH2OH
CH3CH2OH![]() CH3CHO
CH3CHO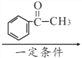
![]()
![]()
 ��
��

����Ŀ������β����Ҫ����CO2 ��CO��SO2��NOx �����ʣ�β����CO����������(NOx) ����Ӱ�����ǵ�����ͽ�������ѧ�����߶Ե�������Ĵ������˹㷺��������о���
��1�����ü��黹ԭNOx
��CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g)��H1=-574kJ/mo l��
��CH4(g)+4NO(g)=2N2(g)+CO2(g)+H2O(g) ��H2=-1160kJ/mo l,
����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ________________________________��
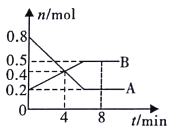
��2������ͬ����CO(g)��H2O(g)�ֱ�ͨ�����Ϊ2L�ĺ����ܱ������У����з�ӦCO(g)+H2O(g)![]() CO2(g)+H2(g)���õ������������ݣ�
CO2(g)+H2(g)���õ������������ݣ�
ʵ���� | �¶��� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
CO | H2O | H2 | CO | |||
1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
2 | 900 | 2 | 1 | 0.4 | 1.6 | 4 |
3 | 900 | a | b | c | d | t |
��ʵ��1����v(CO2) ��ʾ�ķ�Ӧ����Ϊ/span>_________(������λ��Ч���֣���ͬ)��
���÷�ӦΪ________(��������������������) ��Ӧ��ʵ��2�����µ�ƽ�ⳣ��K=________��
������ƽ��״̬ʱ��ʵ��2 ��ʵ��3�и����ʵ����������ֱ���ȣ���t��4min,��a��bӦ����Ĺ�ϵ��_________________________(�ú�a��b����ѧʽ��ʾ)��
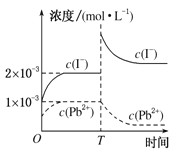
��3��CO�����ǵĴ������ɲⶨ����β���Ƿ�����ŷű����÷����ǵĹ���ԭ��������ȼ�ϵ�أ����е������������(Y2O3) �������(ZrO2) ���壬�ܴ���O2-�����ĵ缫��ӦʽΪ____________��
��4��SO2���øƻ�����Ӧ��ȥ����Ӧ���ɵ�CaSO4��һ�������ʣ���Ksp=9.0��10-6������Ũ��Ϊ2��10-3mol/L��Na2SO4��Һ��������CaCl2��Һ����������ɳ�������CaCl2��Һ����СŨ��Ϊ_____________��