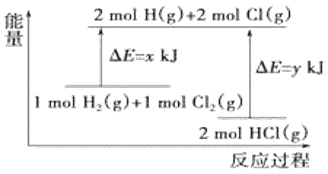
��Ŀ����
����Ŀ����֪ 2CH3OH(g)![]() C2H4(g)+2H2O(g)�� ij�о�С�齫�״�������һ�������ٳ���ͨ����ͬ����ͬ�ִ����� ��ͬ�¶ȵõ�����ͼ�� �����н��۲���ȷ����
C2H4(g)+2H2O(g)�� ij�о�С�齫�״�������һ�������ٳ���ͨ����ͬ����ͬ�ִ����� ��ͬ�¶ȵõ�����ͼ�� �����н��۲���ȷ����
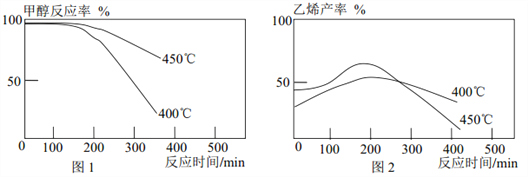
A. һ��ʱ���״���Ӧ���½������Ǵ��������½�
B. �ۺ�ͼ 1�� ͼ 2 ��֪�� �״���������������Ӧ
C. ���ı�״����������٣� ����Ӱ��״���Ӧ�ʺ���ϩ����
D. ����ϩ�Ƚ����˵��¶��� 450������
���𰸡�C
��������A. �������������¶�Ӱ��ģ��¶�̫�ߴ�����ʧȥ���Եģ���A��ȷ�� B���� 2CH3OH(g)![]() C2H4(g)+2H2O(g)ͼ1֪450��ʱ�״��ķ�Ӧ���ʱ�400���죬ͼ2 450��C2H4(g)�IJ��ʵ���400���IJ��ʣ�������������Ӧ��������B��ȷ��C. ���ı�״����������٣��״���Ӧ�ʺ���ϩ���ʶ����ı䡣��C����D. ��ͼ1֪450����������ϩ���ʱȽϸ�ߡ���D��ȷ���𰸣�C��
C2H4(g)+2H2O(g)ͼ1֪450��ʱ�״��ķ�Ӧ���ʱ�400���죬ͼ2 450��C2H4(g)�IJ��ʵ���400���IJ��ʣ�������������Ӧ��������B��ȷ��C. ���ı�״����������٣��״���Ӧ�ʺ���ϩ���ʶ����ı䡣��C����D. ��ͼ1֪450����������ϩ���ʱȽϸ�ߡ���D��ȷ���𰸣�C��

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�����Ŀ����Ҫ��ش��������⣺
��1��ʵ������ͨ����NaOH��Һ����ϴ�����ᴿ������100mL3mol��L-1��NaOH��Һ���ձ�״����4.48LCO2ʱ��������Һ�и�����Ũ���ɴ�С��˳��Ϊ__��
��2�������£���һ�������0.1mol��L-1�Ĵ�����Һ�м�ˮϡ�ͺ�����˵����ȷ����__(����ĸ)��
A.��Һ�е������ӵ���Ŀ����
B.����ĵ���̶�����c(H+)Ҳ����
C.��Һ��![]() ����
����
D.��Һ��![]() ��С
��С
��3���ٳ����½�0.15mol��L-1��ϡ����V1mL��0.1mol��L-1��NaOH��ҺV2mL��ϣ�������Һ��pHΪ1����V1��V2=__(��Һ����ı仯���Բ���)��
�ڳ���������Һ��pH=3��HA��ҺV1mL��pH=11��NaOH��ҺV2mL��϶��ã�������˵����ȷ����__(����ĸ)��
A.����Ϻ���Һ�����ԣ���c(H+)+c(OH-)=2��10-7mol��L-1
B.��V1=V2����Ϻ���Һ��pHһ������7
C.����Ϻ���Һ�����ԣ���V1һ������V2
D.����Ϻ���Һ�ʼ��ԣ���V1һ��С��V2
��4�������£�Ũ�Ⱦ�Ϊ0.1mol��L-1������������Һ��pH�����ʾ��
���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
�ٸ��ݱ������ݣ���Ũ�Ⱦ�Ϊ0.01mol��L-1���������������Һ�ֱ�ϡ��100����pH�仯��С����__(����ĸ)��
A.HCN B.HClO C.H2CO3 D.CH3COOH
�ڸ����������ݣ��ж����з�Ӧ���Գ�������__(����ĸ)��
A.CH3COOH+Na2CO3=NaHCO3+CH3COONa
B.CH3COOH+NaCN=CH3COONa+HCN
C.CO2+H2O+2NaClO=Na2CO3+2HClO
D.NaHCO3+HCN=NaCN+H2O+CO2��
��5���������ӿ�ʼ����ʱ��pH�����ʾ��
���� | Fe2+ | Cu2+ | Mg2+ |
pH | 7.6 | 5.2 | 10.4 |
������ͬŨ��Cu2+��Mg2+��Fe2+����Һ�еμ�NaOH��Һʱ��__(�����ӷ���)�ȳ�����
����Ŀ��ʵ���ҳ����ü�ȩ���ⶨ(NH4)2SO4��Ʒ�е��������������䷴Ӧԭ��Ϊ��4NH![]() +6HCHO=3H++6H2O+(CH2)6N4H+[�ζ�ʱ��1mol(CH2)6N4H+��1molH+�൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣij��ȤС���ü�ȩ������������ʵ�飺
+6HCHO=3H++6H2O+(CH2)6N4H+[�ζ�ʱ��1mol(CH2)6N4H+��1molH+�൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣij��ȤС���ü�ȩ������������ʵ�飺
�����ȡ��Ʒ1.500g��
�������Ʒ�ܽ����ȫת�Ƶ�250mL����ƿ�У����ݣ����ҡ�ȡ�
�������ȡ25.00mL��Ʒ��Һ��250mL��ƿ�У�����10mL20%�����Լ�ȩ��Һ��ҡ�ȡ�����5min����1��2�η�̪��Һ����NaOH����Һ�ζ����յ�.�����������������ظ�2�Ρ�
��1�����ݲ������գ�
�ٵζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�___��
�ڴﵽ�ζ��յ�ı�־��___��
�����²�����ɲ����Ʒ�е�����������ƫ�ߵ�ԭ�������___��
A.���Ʊ���Һ�����������л���Na2CO3����
B.�ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ
C.ʢװδ֪Һ����ƿ������ˮϴ����ˮδ����
D.�ζ����յ����ʱ���ֵζ��ܼ��촦����һ����Һ
E.δ�ñ�Һ��ϴ��ʽ�ζ���
��2���ζ�������±���ʾ��
�ζ����� | ������Һ�����/mL | ����Һ����� | |
�ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
1 | 25.00 | 1.02 | 21.03 |
2 | 25.00 | 2.00 | 21.99 |
3 | 25.00 | 0.20 | 20.20 |
��NaOH����Һ��Ũ��Ϊ0.1010mol/L�������Ʒ�е�����������Ϊ__��