��Ŀ����
����Ŀ����������˵����ȷ����__________��
A��Ԫ�صĵ縺��Խ���䵥��Խ�ȶ�
B�����Ӿ����п��ܲ����ڹ��ۼ�
C��������Խ���γɵ����Ӿ���Խ�ȶ�
D��������������Ӿ����������չ��
���������к���C��N��Mn��Ԫ�أ�ʵ���г��ù��������������ⶨ�������̵ĺ�������Ӧԭ��Ϊ2Mn2++5S2O82-+8H2O ![]() 2MnO4-+10SO42-+16H+
2MnO4-+10SO42-+16H+
��1��Mnԭ�ӵļ۲���ӵĹ������ʽ�������Ų�ͼ��Ϊ____________________��
��2����֪H2S2O8�Ľṹ��ʽ��ͼ��ʾ��
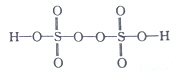
��H2S2O8��S�Ĺ���ӻ���ʽΪ______________��H��O��S����Ԫ���У��縺������Ԫ����___________����Ԫ�ط��ţ���
��S��̬ԭ���е��ӵ��˶�״̬��_________�֡�
��������Ӧ��S2O82-���ѵĹ��ۼ�����Ϊ___________����������������������) ��ÿ����1mol MnO4-�����ѵĹ��ۼ���ĿΪ___________NA��
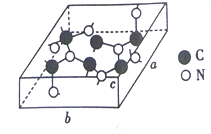
��3��C��N���γɶ��ֽṹ�ľ��塣һ�����͵ij�Ӳ���������ڽ��ʯ�Ľṹ����Ӳ�ȱȽ��ʯ���侧����ͼ��ʾ��ͼʾԭ�Ӷ������ھ����ڣ����仯ѧʽΪ______________����֪��������a=0.64nm��b=0.55nm��c=0.24nm����þ�����ܶ�Ϊ_______________���г�ʽ�Ӽ��ɣ���ʽ���в�������ĸ��g/cm3��
���𰸡� BC ![]() sp3 O 16 ���� 2.5 C3N4
sp3 O 16 ���� 2.5 C3N4 ![]()
�������������������A�������ȶ�������ӽṹ�йأ�B��ϡ�����幹�ɵķ��Ӿ����в����ڹ��ۼ���C��������Խ�����Ӽ�����Խ����D�����Ӿ��岻��������չ����
������1��Mn��25��Ԫ�أ��۵��ӵĵ����Ų�ʽ��![]() �����ݺ��ع��������������ԭ����дMn�۲���ӵĹ������ʽ��
�����ݺ��ع��������������ԭ����дMn�۲���ӵĹ������ʽ��
��2�����ݼ۲���Ӷ�=�� �����Ӷ�+����ԭ���ϵŵ��Ӷԣ����S2O8 2-�Ľṹ�ж�Sԭ���ӻ�����;�ǽ�����Խǿ�縺��Խ����
ԭ�Ӻ�����һ�����Ӿ���1���˶�״̬��
��Ӧ��S2O82-���ѵĹ��ۼ���O-O���������������������������ӷ���ʽÿ����2mol MnO4-������5mol S2O82-��
��3��ͼʾԭ�Ӷ������ھ����ڣ�����ͼʾ��ÿ����������6��Cԭ����8��Nԭ����ÿ��������������![]() ��ÿ�������������
��ÿ�������������![]() ������
������![]() �����ܶȣ�
�����ܶȣ�
��������A�������ȶ�������ӽṹ�й�����F�ĵ縺�Դ���N��N2��F2�ȶ�����A������B��ϡ�����幹�ɵķ��Ӿ����в����ڹ��ۼ�����B��ȷ��C��������Խ�����Ӽ�����Խ��������Խ�ȶ�����C��ȷ��D�����Ӿ��岻��������չ������D������
������1��Mn��25��Ԫ�أ��۵��ӵĵ����Ų�ʽ��![]() �����ݺ��ع��������������ԭ���� Mn�۲���ӵĹ������ʽ��
�����ݺ��ع��������������ԭ���� Mn�۲���ӵĹ������ʽ��![]() ��
��
��2������ԭ�Ӽ۲���Ӷ���=�� �����Ӷ�+����ԭ���ϵŵ��Ӷ�=4+12(6-4��1-2)=4������Sԭ�Ӳ�ȡsp3�ӻ����ǽ�����Խǿ�縺��Խ����H��O��S����Ԫ���У��縺������Ԫ����O��
����һ�����Ӿ���1���˶�״̬��Sԭ�Ӻ�����16�����ӣ�����S��̬ԭ���е��ӵ��˶�״̬��16����
����Ӧ��S2O82-���ѵĹ��ۼ���O-O��������������������S2O82-���ѵĹ��ۼ�����Ϊ�������������ӷ���ʽÿ����2mol MnO4-������5mol S2O82-��ÿ����1mol MnO4-�����ѵ�S2O82-�й��ۼ���ĿΪ2.5 NA��
��3��ͼʾԭ�Ӷ������ھ�����������ÿ����������6��Cԭ����8��Nԭ������ѧʽΪC3N4��ÿ��������������![]() ��ÿ�������������
��ÿ�������������![]() ������
������![]() ���ܶ�=
���ܶ�= ![]() ��
��![]() =
=![]() g/cm3��
g/cm3��