��Ŀ����
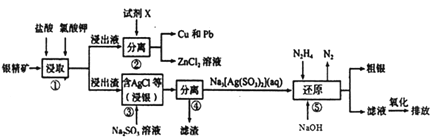
����Ŀ����һ�ֺ�����(��Ag��Zn��Cu��Pb������SiO2)����ȡAg��Cu��Pb�Ĺ�����������:
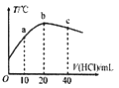
(1)��������߿����н������ӽ�ȡ�ʣ����ɸı������Ũ�Ⱥ�����ص����⣬���ɲ�ȡ�Ĵ�ʩ��__________(д�����ּ���)
(2)���������Լ�XΪ______ (�ѧʽ����ͬ)�������������ijɷ�Ϊ_______��
(3)������������Ӧ�Ļ�ѧ����ʽΪ____________________��
(4)������������Ӧ�����ӷ���ʽΪ____________________������N2H4(��) �ĵ���ʽΪ____��
(5)����ԭ���������Һ�������������е�������ҪΪ_______��
(6) ��֪�����£�Ksp(AgCl)=1.8��10-10��Ksp(AgI)=1.0��10-16������AgC1������Һ�м���NaCl ���壬AgCl �������ܽ��_____ (������������������������������)����AgCl ������Һ�еμ�NaI��Һ����AgCl ��ʼת��ΪAgIʱ��I-��Ũ�ȱ��벻����_____mol/L (���û���)��
���𰸡� �ʵ���߽�ȡ�¶ȡ��ʵ��ӳ���ȡʱ�䡢���衢��������� Zn SiO2 AgCl+2Na2SO3==Na3[Ag(SO3)2]+NaCl 4[Ag(SO3)2]3-+N2H4+4OH-=4Ag��+8SO32-+N2��+4H2O 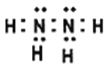 Na2SO4 ����
Na2SO4 ���� ![]()
��������������(�仯ѧ�ɷ��У�Ag��Zn��Cu��Pb������SiO2��)�����������ؽ�ȡ�����ˣ���Һ�к���Zn2+��Cu2+��Pb2+�������к���SiO2��AgCl�ȣ�����Һ�мӽ�����ԭ��Zn����Cu2+��Pb2+��ԭΪ���ʣ����Լ�XΪZn�����������Ϊ���ˣ��õ�Cu��Pb��ZnCl2��Һ������������SiO2��AgCl����Na2SO3��Һ��AgCl��Na2SO3��Ӧ����Na3[Ag(SO3)2]��NaCl�����ˣ�����ΪSiO2����ҺΪNa3[Ag(SO3)2]��NaCl������Һ�м�N2H4����������������Ag�͵�������Һ�к����������ƣ��������������ƺ��ŷ���
(1)Ϊ��߽�ȡ�ʿɲ�ȡ�Ĵ�ʩ�����ɸı������Ũ�Ⱥ�����ص����⣬�������ʵ���ȡ��ȡ�¶Ȼ��ʵ��ӳ���ȡʱ����ֽ���ȣ��ʴ�Ϊ���ʵ���ȡ��ȡ�¶Ȼ��ʵ��ӳ���ȡʱ����ֽ��裻
(2)�����̷�����֪���Լ�XΪZn������������г����������⣬�����еijɷ���SiO2��
�ʴ�Ϊ��Zn��SiO2��
(3)����ۼ�Na2SO3��Һ��AgCl��Na2SO3��Ӧ����Na3[Ag(SO3)2]��NaCl����Ӧ�Ļ�ѧ����ʽΪAgCl+2Na2SO3=Na3[Ag(SO3)2]+NaCl���ʴ�Ϊ��AgCl+2Na2SO3=Na3[Ag(SO3)2]+NaCl��
(4)��ҺΪNa3[Ag(SO3)2]��NaCl������Һ�м�N2H4��Na3[Ag(SO3)2]��N2H4��Ӧ����Ag�͵������������ƣ���Ӧ�����ӷ���ʽΪ��[Ag(SO3)2]3-+N2H4+4OH-=4Ag��+8SO32-+N2��+4H2O������N2H4(��)�ĵ���ʽΪ![]() ���ʴ�Ϊ��[Ag(SO3)2]3-+N2H4+4OH-=4Ag��+8SO32-+N2��+4H2O��
���ʴ�Ϊ��[Ag(SO3)2]3-+N2H4+4OH-=4Ag��+8SO32-+N2��+4H2O��![]() ��
��
(5)��Һ�к����������ƣ��������������ƣ�������ԭ��Һ�����������ŷ�Һ�����ʵ���Ҫ�ɷ�ΪNa2SO4���ʴ�Ϊ��Na2SO4��
(6)��AgC1������Һ�м���NaCl���壬������Ũ������ʹAgCl���ܽ�ƽ�������ƶ���AgCl�������ܽ�Ƚ��ͣ���AgCl������Һ��c(Cl-)= c(Ag+)=![]() =
=![]() mol/L���μ�NaI��Һ����AgCl��ʼת��ΪAgIʱ��������c(Ag+) c (I-)��Ksp(AgI)�����c (I-)��
mol/L���μ�NaI��Һ����AgCl��ʼת��ΪAgIʱ��������c(Ag+) c (I-)��Ksp(AgI)�����c (I-)��![]() =
=![]() =
=![]() ��10-11���ʴ�Ϊ��������
��10-11���ʴ�Ϊ��������![]() ��10-11��
��10-11��

 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д�