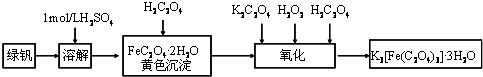
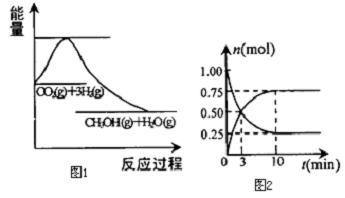
��Ŀ����
18�������������ѵ���Ҫ������Ѱף������Ķ������ѣ���һ�������ʸߡ���ɫ�����ڸ���ǿ����ѧ�����ȶ��İ�ɫ���ϣ�����������Ҫ�ɷ�FeTiO3����Fe2O3��SiO2�����ʣ���ȡ�������ѣ��������ᷨ�����������£�
��1����������Ҫ�ɷ���Ũ���ᷴӦ����Ҫ������TiOSO4��FeSO4���÷�Ӧ�Ļ�ѧ����ʽFeTiO3+2H2SO4�TFeSO4+TiOSO4+2H2O��
��2��Ϊ��ߡ������ۿ顱ˮ��ȡʱ�Ľ����ʣ����˲���ѭ����ȡ���ӳ�ʱ�䡢�ۿ�����⣬���˵�����������ѡ���������衢�ʵ������¶ȣ���дһ�֣���
��3������ʱ���貣������Ϊ©�������������ձ�������Һ�еõ�XΪ�̷���FeSO4•7H2O�������ʵ���������Ϊ������Ũ������ȴ�ᾧ������ϴ�ӡ����¸��ʵ����Ҫ���ơ��������Һʱ���������м��Ŀ���Ƿ�ֹFe2+��������
��4���ڢ۲���Ӧ��ѧ����ʽTiOSO4+2H2O=H2TiO3+H2SO4��
��5����ʵ��ʱ��Ҫ450mL2mol/L��NaOH��Һ�����ھ�ȷ����ʱ������������ƽ��ȡNaOH����40.0g����ʹ�õ�������������ƽ����Ͳ���ձ�������������ͷ�ιܡ�ҩ���⣬��������500ml����ƿ�����������ƣ���
���� ��������Ҫ�ɷ�FeTiO3����Fe2O3��SiO2�����ʣ�����Ũ������ȷ�Ӧ�õ������ۿ飬����ˮˮ�����õ���ȡҺ�м�����м��ֹ�������ӱ�����Ϊ�����ӣ����˵õ�X����ΪΪ�̷���FeSO4•7H2O���������ҺTiOSO4������ˮ��Ӧ����H2TiO3���������յõ��������ѣ�
��1����������Ҫ�ɷ�FeTiO3��Ũ���ᷴӦ����Ҫ������TiOSO4��FeSO4�����ԭ���غ���ƽ��д��ѧ����ʽ��
��2��Ϊ��ߡ������ۿ顱ˮ��ȡʱ�Ľ����ʿ����������裬�����¶ȣ�ѭ����ȡ���ӳ�ʱ�䡢�ۿ����ȣ�
��3�����������ױ���������������Ϊ�����ӣ������������ӱ������Ҳ��������ʣ�������м���Է�ֹ�������ӱ�������
��4���ڢ۲���Ӧ��TiOSO4����ˮ��Ӧ����H2TiO3������ķ�Ӧ��
��5����ʵ��ʱ��Ҫ450mL2mol/L��NaOH��Һ��ʵ��������450ml����ƿ����Ҫ��500ml����ƿ�н������ã�Ȼ��ȡ��450ml��Һ�����ھ�ȷ����ʱ��Ҫ�������������500ml��Һ���㣬
��� �⣺��������Ҫ�ɷ�FeTiO3����Fe2O3��SiO2�����ʣ�����Ũ������ȷ�Ӧ�õ������ۿ飬����ˮˮ�����õ���ȡҺ�м�����м��ֹ�������ӱ�����Ϊ�����ӣ����˵õ�X����ΪΪ�̷���FeSO4•7H2O���������ҺTiOSO4������ˮ��Ӧ����H2TiO3���������յõ��������ѣ�
��1����������Ҫ�ɷ�FeTiO3��Ũ���ᷴӦ����Ҫ������TiOSO4��FeSO4�����ԭ���غ���ƽ��д��ѧ����ʽΪ��FeTiO3+2H2SO4�TFeSO4+TiOSO4+2H2O��
�ʴ�Ϊ��FeTiO3+2H2SO4�TFeSO4+TiOSO4+2H2O��
��2��Ϊ��ߡ������ۿ顱ˮ��ȡʱ�Ľ����ʣ����˵���������ѡ���������裬�����¶ȣ�ѭ����ȡ���ӳ�ʱ�䡢�ۿ����ȣ�
�ʴ�Ϊ���������衢�ʵ������¶ȣ�
��3�����������ױ���������������Ϊ�����ӣ������������ӱ������Ҳ��������ʣ�������м���Է�ֹ�������ӱ�������2Fe3++Fe=3Fe2+��
�ʴ�Ϊ��2Fe3++Fe=3Fe2+��
��4���ڢ۲���Ӧ��TiOSO4����ˮ��Ӧ����H2TiO3������ķ�Ӧ �Ļ�ѧ����ʽΪ��TiOSO4+2H2O=H2TiO3+H2SO4��
�ʴ�Ϊ��TiOSO4+2H2O=H2TiO3+H2SO4��
��5����ʵ��ʱ��Ҫ450mL2mol/L��NaOH��Һ��ʵ��������450ml����ƿ����Ҫ��500ml����ƿ�н������ã�Ȼ��ȡ��450ml��Һ�����ھ�ȷ����ʱ��Ҫ�������������500ml��Һ���㣬����������ƽ��ȡNaOH��������=0.5L��2mol/L��40g/mol=40.0g����ʹ�õ�������������ƽ����Ͳ���ձ�������������ͷ�ιܡ�ҩ���⣬�������� 500ml����ƿ��
�ʴ�Ϊ��40.0��500ml����ƿ��
���� ���⿼���������ᴿ�����롢�Ʊ������̷����жϣ�һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ����״���Ϊ�������Ƶ����������ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | ʯ������ϡ���ᷴӦ��OH-+H+�TH2O | |
| B�� | ��ϡ�����ȥͭ�̣�4H++Cu2��OH��2CO3�T2Cu2++CO2��+3H2O | |
| C�� | NaHCO3��Һ�еμӹ�����Ba��OH��2��Һ��2HCO3-+2OH-+Ba2+�TBaCO3��+CO32-+2H2O | |
| D�� | ���������Ũ�ȵ�Ca��HCO3��2��Һ��NaOH��Һ��ӦCa2++2HCO3-+2OH-�TCaCO3��+2H2O+CO32- |
| A�� | �������̾���ǿ�����ԣ�������H2O2�ֽ�������� | |
| B�� | Ũ�����ڳ�������ʹ���ۻ����������۳�����Ũ���� | |
| C�� | K2FeO4���л�ԭ�ԣ�����������ˮ��ɱ������ | |
| D�� | SO2����Ư���ԣ���ʹ��ɫKMnO4��Һ��ɫ |
| A�� | ���������ͨ��ʢ������KMnO4��Һ��ϴ��ƿ | |
| B�� | ���������ͨ��ʢ��������ˮ��ϴ��ƿ | |
| C�� | �����������ͨ����������Ni�����ȵ������·�Ӧ | |
| D�� | ���������ͨ��ʢ��NaOH��Һ��ϴ��ƿ |
| A�� | aΪ0.896 | B�� | ��0.16mol HNO3����ԭ | ||
| C�� | ��Ӧ����Һ��c��NO3-��Ϊ0.98mol/L | D�� | ��Ӧ��ת�Ƶ���0.4mol |
| A�� | ��������茶�����˺�����ˮ�Ҵ�ϴ�ӣ���˾ƥ�־�����˺���ˮϴ�� | |
| B�� | �����еİ�Ϣ�����ơ������Ƶ�����ˮ��ˮ��ʹ��Һ������ | |
| C�� | Ҫ����Һ�н�MnO4-��ȫת��ΪMn2+����������KMnO4��Һ�еμ�H2O2��Һ����ɫ��ȫ��ʧ | |
| D�� | �������ķ�������֬������Ӧ�Ļ��Һ�з������֬������ | |
| E�� | ������ķ�����ȥNa+��SO42-��Cl-������ |