��Ŀ����
ij��ѧС���ͬѧ��Na2CO3��NaCl�Ĺ���������Na2CO3��������������̽��ʵ�飬�����������ʵ�鰸--�����������
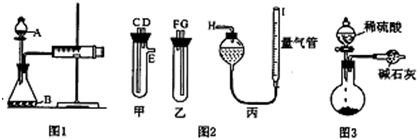
��1����ͬѧ��ͼ1��ʾװ�ã���x g�����������ϡ���ᷴӦ�ⶨ������CO2����������ʵ�鿪ʼʱ����װ�������Եķ����� ��
��2����ͬѧ��ͼ2����ͼ1�еķ������ռ�װ�ã��ס������Թܸ����������ܣ��������Ӷ�Ӧ�ӿں���ʢϡ������Թܣ�������Ӧ���ų����壬��Na2CO3��NaCl�Ĺ��������ϡ����Ӧ�ֱ����� �� �����У�����ң��������ס��ҽӿڵ����ӷ�ʽ���£�C���� ��D���� ��E���� ����д���ӿڵı�ţ���
��3����ͬѧ��ͼ3װ�òⶨCO2����������װ�ô�������ȱ�ݣ��Ӷ�����ʵ���������������ʹ�ⶨ���ƫС����Ҫԭ�� ��
��4����ͬѧ��ȡ2.0g Na2CO3��NaCl�Ĺ����������ͼ2װ�ý���ʵ�飬����ʱӦע�⣺
�ٽ�ʵ��װ�ûָ������£��� ���������밼Һ����ʹ���ƽ������������ϡ������ռ������������Ϊ224mL����״���£�����û������Na2CO3��������Ϊ ��

��1����ͬѧ��ͼ1��ʾװ�ã���x g�����������ϡ���ᷴӦ�ⶨ������CO2����������ʵ�鿪ʼʱ����װ�������Եķ�����
��2����ͬѧ��ͼ2����ͼ1�еķ������ռ�װ�ã��ס������Թܸ����������ܣ��������Ӷ�Ӧ�ӿں���ʢϡ������Թܣ�������Ӧ���ų����壬��Na2CO3��NaCl�Ĺ��������ϡ����Ӧ�ֱ�����
��3����ͬѧ��ͼ3װ�òⶨCO2����������װ�ô�������ȱ�ݣ��Ӷ�����ʵ���������������ʹ�ⶨ���ƫС����Ҫԭ��
��4����ͬѧ��ȡ2.0g Na2CO3��NaCl�Ĺ����������ͼ2װ�ý���ʵ�飬����ʱӦע�⣺
�ٽ�ʵ��װ�ûָ������£���
��������1�������װ�������ԣ��ɸ��ݸ÷�Ӧ�������ص㣬��������ע���������ķ�����ɣ�
��2����ͼװ�ÿ�֪����c֧�ܣ�ӦΪ��������װ�ã����Ӧ����Na2CO3��NaCl�Ĺ�������ҷ������ᣬ���ӵ���ʱ��Ӧ��C����F��D����G��E����H��
��3�����ݼ�ʯ�ҵijɷֿ�֪����ʯ�Ҽȿ����ն�����̼Ҳ������ˮ��ͬʱ���ɵĶ�����̼��Ӧ����װ���ڻ��в������Լ������������Ӵ��Ƚ��з������ɣ�
��4�������������ܵĶ���ע������ش𣬽�ʵ��װ�ûָ������£�ʹ����������������Һ����ƽ�������밼Һ����ʹ���ƽ��
������̼���ƺ�ϡ���ᷴӦ���ɵĶ�����̼����������ʵ��������̼Ԫ���غ����̼���������õ�̼���Ƶĺ�����
��2����ͼװ�ÿ�֪����c֧�ܣ�ӦΪ��������װ�ã����Ӧ����Na2CO3��NaCl�Ĺ�������ҷ������ᣬ���ӵ���ʱ��Ӧ��C����F��D����G��E����H��
��3�����ݼ�ʯ�ҵijɷֿ�֪����ʯ�Ҽȿ����ն�����̼Ҳ������ˮ��ͬʱ���ɵĶ�����̼��Ӧ����װ���ڻ��в������Լ������������Ӵ��Ƚ��з������ɣ�
��4�������������ܵĶ���ע������ش𣬽�ʵ��װ�ûָ������£�ʹ����������������Һ����ƽ�������밼Һ����ʹ���ƽ��
������̼���ƺ�ϡ���ᷴӦ���ɵĶ�����̼����������ʵ��������̼Ԫ���غ����̼���������õ�̼���Ƶĺ�����
����⣺��1�������װ�������ԣ��ɸ��ݸ÷�Ӧ�������ص㣬��������ע���������ķ�����ɣ��������Ϊ�ر�A����������ע������������һ�����룬һ��ʱ����ɿ��������������ܻص�ԭλ��֤����©��������©����
�ʴ�Ϊ���ر�A����������ע������������һ�����룬һ��ʱ����ɿ��������������ܻص�ԭλ��֤����©��������©����
��2����ͼװ�ÿ�֪����c֧�ܣ�ӦΪ��������װ�ã����Ӧ����Na2CO3��NaCl�Ĺ�������ҷ������ᣬGΪ����װ�ã����ݹ��죬���ü�ʽ�ζ��ܴ��棬���ӵ���ʱ��Ӧ��C����F��D����G��E����H��
�ʴ�Ϊ���ף��ң�F��G��H��
��3����������CO2�����л���ˮ����������е�CO2��ˮ�������������У��ᵼ������ƫ��ʹ�ⶨ�������ƫС����Ҫԭ��װ���е�CO2û��ȫ������ʯ�����գ�Ӧͨ�뵪���ɽ������ų���
�ʴ�Ϊ��װ���е�CO2û��ȫ������ʯ�����գ�
��4�����������ܵĶ���ע������ش𣬽�ʵ��װ�ûָ������£�ʹ����������������Һ����ƽ�������밼Һ����ʹ���ƽ��
����������ϡ������ռ������������Ϊ224mL����״���£������ʵ���=
=0.01mol����û������Na2CO3���ʵ���Ϊ0.01mol��̼������������=
��100%=53%��
�ʴ�Ϊ��ʹ����������������Һ����ƽ��53%��
�ʴ�Ϊ���ر�A����������ע������������һ�����룬һ��ʱ����ɿ��������������ܻص�ԭλ��֤����©��������©����
��2����ͼװ�ÿ�֪����c֧�ܣ�ӦΪ��������װ�ã����Ӧ����Na2CO3��NaCl�Ĺ�������ҷ������ᣬGΪ����װ�ã����ݹ��죬���ü�ʽ�ζ��ܴ��棬���ӵ���ʱ��Ӧ��C����F��D����G��E����H��
�ʴ�Ϊ���ף��ң�F��G��H��
��3����������CO2�����л���ˮ����������е�CO2��ˮ�������������У��ᵼ������ƫ��ʹ�ⶨ�������ƫС����Ҫԭ��װ���е�CO2û��ȫ������ʯ�����գ�Ӧͨ�뵪���ɽ������ų���
�ʴ�Ϊ��װ���е�CO2û��ȫ������ʯ�����գ�
��4�����������ܵĶ���ע������ش𣬽�ʵ��װ�ûָ������£�ʹ����������������Һ����ƽ�������밼Һ����ʹ���ƽ��
����������ϡ������ռ������������Ϊ224mL����״���£������ʵ���=
| 0.224L |
| 22.4L/mol |
| 0.01mol��106g/mol |
| 2.0g |
�ʴ�Ϊ��ʹ����������������Һ����ƽ��53%��
���������⿼�����ʵĺ����ⶨ��ʵ����ƣ������ڿ���ѧ������������ʵ�������ͽ�����������Ŀ��Ϊ�ۺϣ�Ϊ�߿��������ͣ�ע�����ʵ��ԭ���Ͳ�����������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
�����Ŀ
 C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________ 
 C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________