��Ŀ����
���û�ѧ��Ӧԭ���о��⡢�����ȡ���ȵ��ʼ��仯����ķ�Ӧ����Ҫ����
��1����֪��25��ʱKSP��AgCl��=1.6��l0��10 KSP��AgI��=1.5��l0��16
��ˮ�к��д�����Ԫ�أ�����Ԫ�����ȣ���Ԫ����⣬���ں�ˮ�о��Ի���̬���ڡ���25���£���0.1L0.002mol��L��l��NaCl��Һ�м���0.1L0.002mol��L��l��������Һ���а�ɫ�������ɣ�����������ԭ���ǣ�ͨ������ش� ����Ӧ��Ļ���Һ�м�������0.1L0.002mol��L��1��NaI��Һ�������������� �������������ԭ���ǣ������ӷ���ʽ��ʾ�� ��
��2������������Ʊ������ܶ࣬���з�����ԭ����������ߵ��� ������ţ���
A��BaO2 + H2SO4�� BaSO4 �� + H2O2
B��2NH4HSO4 (NH4)2S2O8 + H2��
(NH4)2S2O8 + H2��
(NH4)2S2O8 + 2H2O �� 2NH4HSO4 + H2O2
C��CH3CHOHCH3 + O2�� CH3COCH3 + H2O2
D���һ�����������ͼ
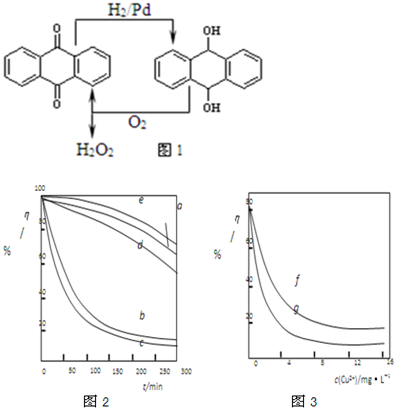
��3��ij���ױ����˲�ͬ�������Ӽ���Ũ�ȶ�˫��ˮ�������⺣��������Һ��Ӧ���ʵ�Ӱ�죬ʵ������ͼ1��ͼ2��ʾ��
ע������ʵ������¶�Ϊ20�桢w(H2O2)��0.25%��pH��7.12������������ҺŨ��Ϊ8mg��L�D1�������½��С�ͼ1������a��H2O2��b��H2O2+Cu2+��c��H2O2+Fe2+��d��H2O2+Zn2+��e��H2O2+Mn2+��ͼ2������f����Ӧʱ��Ϊ1h��g����Ӧʱ��Ϊ2h����ͼ�е��������������������Һ��ճ�ȣ���������Ũ������Һճ������أ���
��������Ϣ��֪����������������� ������ţ���
A����������ʹ�ý��ⷴӦ���ʼ���
B���������ӶԸý��ⷴӦ�Ĵ�Ч�ʱ�ͭ���ӵ�
C������������Һճ�ȵı仯�����ɷ�ӳ���併�ⷴӦ���ʵĿ���
D��һ�������£�ͭ����Ũ��һ��ʱ����Ӧʱ��Խ��������������ҺŨ��ԽС
��10�֣�
��1��Q (AgCl)= c(Ag+)��c (Cl��)= 1��l0��6��Ksp(AgCl) ��2�֣�
��ɫ����ת��Ϊ��ɫ���� ��2�֣�
AgCl(s) + I����aq�� == AgI(s) + Cl��(aq) (2�֣�����s��1�֣�����aq���۷�)
��2��D��2�֣�
��3��B��2�֣�
���������������1��Q (AgCl)= c(Ag+)��c (Cl��)= 1��l0��6��Ksp(AgCl)��KSP��AgI��=1.5��l0��16>Q (AgCl),�ʰ�ɫ����ת��Ϊ��ɫ���������ӷ���ʽ�ǣ�AgCl(s) + I����aq�� == AgI(s) + Cl��(aq)��
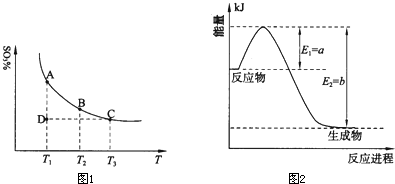
��2��ԭ����������ߵ��Ƿ�Ӧ�ﶼת��Ϊ���������Щ��Ӧ�������±�Ϊ�������ٷ�Ӧ���ʴ�D��
��3��B�H2O2���������Զ�Fe2+���л�ԭ�ԣ�֮�䷢����������ԭ��Ӧ�����������ӶԸý��ⷴӦ�Ĵ�Ч�ʱ�ͭ���ӵ�˵���Ǵ���ġ���ѡB��
���㣺 ���ܵ���ʵ��ܽ�ƽ�� �ܶȻ��ļ��� ͼ�����
���������⿼�黯ѧƽ�⡢�Ȼ�ѧ����ʽ������ת������ͼ�������Ѷ��еȣ�ѧ��ע��Ի���֪ʶ��ѧϰ��Ӧ�á�

 ���û�ѧ��Ӧԭ���о������ȡ���ȵ��ʼ��仯����ķ�Ӧ����Ҫ���壮
���û�ѧ��Ӧԭ���о������ȡ���ȵ��ʼ��仯����ķ�Ӧ����Ҫ���壮