��Ŀ����
����˵����ȷ����
- A.�ڱ�״���£�22.4L SO3�����е���ԭ����Ϊ3��6.02��1023
- B.�ڱ�״���£�22.4LNO��NO2�Ļ�����У������е�ԭ�ӵĸ���Ϊ6.02��1023
- C.20mL0.1mol��L��1NaCl��10mL0.1mol��L��1BaCl2��Һ�����������ʵ���Ũ�����
- D.��Na2O2��ˮ�ķ�Ӧ�У�����1mol O2ת�Ƶĵ�����Ϊ4��6.02��1023
B
A������£�SO3Ϊ��̬��
C��ǰ��������Ũ��Ϊ0.1mol/L,����Ϊ0.2mol/L��
D������������ˮ��Ӧ������-1�۱�Ϊ0�ۺ�-2�ۣ���ת�Ƶ�����ĿΪ2��6.02��1023
A������£�SO3Ϊ��̬��
C��ǰ��������Ũ��Ϊ0.1mol/L,����Ϊ0.2mol/L��
D������������ˮ��Ӧ������-1�۱�Ϊ0�ۺ�-2�ۣ���ת�Ƶ�����ĿΪ2��6.02��1023

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ
Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij������ȤС����ʵ��ķ����о���Ӧ���ʵ��й����⣮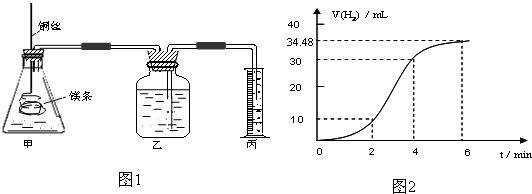
��1��ʵ��1 ̽��Mg�����ᷴӦ���ʵı仯���ɣ�ȡһ��þ������ɰֽ��ȥ���������Ĥ��ͭ˿����þ������װ�ü��У�ʹþ��������ƿ�ڵ����Ϊ2Lϡ���ᣨ�������У�þ�������ᷴӦ����H2������뷴Ӧʱ��Ĺ�ϵ������ͼ2��ʾ��
�ٴ�ͼ2�п���0-6min��ƽ����Ӧ��������ʱ����� ��������ţ�
A��0-2min B��2-4min C��4-6min
�������4-6min ʱ���ڣ���HCl��ʾ��ƽ����Ӧ����Ϊ ��������ͼ2����������ѻ���Ϊ��״���µ����������Һ����仯�ɺ��ԣ�
��ͼ1װ�ü�����þ��������ͭ˿��һ�����ϡ�����жԷ�Ӧ����Ӱ������˵����ȷ����
A���ӿ췴Ӧ���ʵ������������������� B��������Ӧ������������������
C����Ӱ�췴Ӧ���� D���ӿ췴Ӧ���ʵ�����������������С
��2��ʵ��2 ̽����Ũ�ȶ�MnO2��H2O2��Ӧ���ʵ�Ӱ��
��֪MnO2+H2O2+2H+�TMn2++O2��+2H2O����ȡ����MnO2���±��й����ʣ�����ͬ�¶��½���4��ʵ�飬�ֱ��¼�ռ�20.0mL��������ʱ�䣮
���ϱ���V1= mL��V3= mL��
����ͬѧ���ʵ��I������Ϊʵ����ĶԱ�ʵ�飬�������� ��
����ʵ����t2��t3��t4����ɵó���ʵ������� ��

��1��ʵ��1 ̽��Mg�����ᷴӦ���ʵı仯���ɣ�ȡһ��þ������ɰֽ��ȥ���������Ĥ��ͭ˿����þ������װ�ü��У�ʹþ��������ƿ�ڵ����Ϊ2Lϡ���ᣨ�������У�þ�������ᷴӦ����H2������뷴Ӧʱ��Ĺ�ϵ������ͼ2��ʾ��
�ٴ�ͼ2�п���0-6min��ƽ����Ӧ��������ʱ�����
A��0-2min B��2-4min C��4-6min
�������4-6min ʱ���ڣ���HCl��ʾ��ƽ����Ӧ����Ϊ
��ͼ1װ�ü�����þ��������ͭ˿��һ�����ϡ�����жԷ�Ӧ����Ӱ������˵����ȷ����
A���ӿ췴Ӧ���ʵ������������������� B��������Ӧ������������������
C����Ӱ�췴Ӧ���� D���ӿ췴Ӧ���ʵ�����������������С
��2��ʵ��2 ̽����Ũ�ȶ�MnO2��H2O2��Ӧ���ʵ�Ӱ��
��֪MnO2+H2O2+2H+�TMn2++O2��+2H2O����ȡ����MnO2���±��й����ʣ�����ͬ�¶��½���4��ʵ�飬�ֱ��¼�ռ�20.0mL��������ʱ�䣮
| ʵ���� | �� | �� | �� | �� |
| 10%H2O2�����/mL | 5.0 | 5.0 | V1 | V2 |
| 20%��������/mL | 0 | 0.5 | 1.0 | V3 |
| ˮ�����/mL | 15 | 14.5 | V4 | 13.5 |
| ����ʱ��t/s | t1 | t2 | t3 | t4 |
����ͬѧ���ʵ��I������Ϊʵ����ĶԱ�ʵ�飬��������
����ʵ����t2��t3��t4����ɵó���ʵ�������


 �ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�
�ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�