��Ŀ����
��O2��CH4��Na2O2�����ܱ������У���250 ����¶����õ��������ѧ��Ӧ����Ӧֹͣ��ʹ�����ڻָ���250 ����¶ȣ������ڵ���ѹΪ�㡣�ɴ˵ó��Ľ�����ȷ����( )
A.ԭO2��CH4��Na2O2���ʵ���֮��Ϊ1��2��6����Ӧ�����������ɵĹ�����Na2CO3��NaHCO3
B.ԭO2��CH4��Na2O2���ʵ���֮��Ϊ2��1��4����Ӧ�����������ɵĹ�����Na2CO3��NaOH
C.ԭO2��CH4��Na2O2���ʵ���֮��Ϊ1��2��6����Ӧ�����������ɵĹ�����Na2CO3��NaOH
D.ԭO2��CH4��Na2O2���ʵ���֮��Ϊ2��1��4����Ӧ�����������ɵĹ�����NaHCO3��NaOH
C
����:
Na2O2��CO2��H2O��ѭ����Ӧ���տɱ�ʾΪ��Na2O2+CO====Na2CO3��
Na2O2+H2====2NaOH������CH4ȼ�յķ�Ӧʽֻ��д��2CH4+O2��2CO+4H2������6 mol Na2O2���գ���O2��CH4��Na2O2�����ʵ���֮��Ϊ1��2��6���������ɵĹ�����Na2CO3��NaOH��

��ϰ��ϵ�д�
 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ

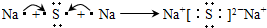
 ��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
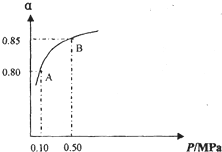
��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��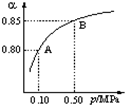 ��2009?��˳ģ�⣩��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
��2009?��˳ģ�⣩��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��