��Ŀ����
����Ŀ���ѱ���Ϊ��������֮��ĵ����������䵥�ʼ��������ں��졢���¡�������ҽ�Ƶ�����������Ҫ��Ӧ�á���ش��������⣺
��1����Ԫ�����ڱ��У��Ԫ������Ԫ��ͬ�壬���Ԫ��λ��__����
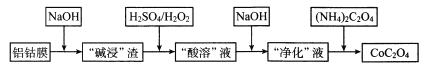
��2��TiO2������Ũ���Ტ����һ�����Ӿ��壬��֪����������������״�ۺ����γɴ��ڵ����������ӣ���ṹ��ͼ��ʾ�����������������Ϊ+n�������������ԭ����Ϊ__��

��3�����ߵ��Ⱦ�Ե�������������ھ�Ե������Ӧ�ù㷺����������������ʯ���ƣ�ÿ��Alԭ����__��Nԭ����������ͬһ��Nԭ��������Alԭ�ӹ��ɵ����幹��Ϊ___����������������___���塣
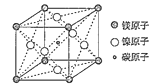
��4��Ti3+���γ���λ��Ϊ6����������ᄃ�壨һ������ɫ��һ������ɫ������ɽ�ΪTiCl36H2O������0.01mol��ɫ����ᄃ���ˮ��Һ�м��������������Һ������0.02molAgCl����������ɫ����ᄃ��Ļ�ѧʽΪ__��
��5��������ͬ�壬���ᾧ����Ƭ��ṹ������һ��Ľṹ��ͼ��ʾ�����ڵķ����������������1mol����ľ�������__mol�����ͼ����ʾ������Ѵ�8ԭ�ӽṹ��ԭ����__����Ԫ�ط��ţ���H3BO3������Bԭ�Ӹ����뼫�Լ�������Ϊ__��
���𰸡�d 0.5n 4 �������� ԭ�Ӿ��� [TiCl��H2O��5]Cl2H2O 3 O 1��6
��������
(1)��Ԫ�����ڱ�����Ԫ�ص�λ���жϣ�
(2)����ͼ֪��ÿ��Tiԭ������Oԭ�Ӹ���=2��![]() =1����Ti��Oԭ�Ӹ���֮��Ϊ1��1��
=1����Ti��Oԭ�Ӹ���֮��Ϊ1��1��
(3)���ݾ���ṹ���۷е�����ж�ȷ����
(4)����Clԭ���غ㣬�����������������������ӣ�1���������ڽ磬��Ϊ����λ����5������ɣ�
(5)1����������γ���6���������ÿ�������2��������ӹ��ã���ԭ�������ֻ��3�����ӣ�����ԭ���γ�3�Թ��õ��Ӷԣ�ÿ��Oԭ���γ�2�����õ��Ӷԡ�ÿ��Hԭ���γ�1�����õ��Ӷԣ�H3BO3�������γ�3��B-O��������3��O-H���Լ���
(1)��Ԫ�����ڱ��У��Ԫ������Ԫ��ͬ�壬Tiλ�ڵ������ڵ�IVB�壬����d���������Ҳλ��d����
(2)����ͼ֪��ÿ��Tiԭ������Oԭ�Ӹ���=2��![]() =1����Ti��Oԭ�Ӹ���֮��Ϊ1��1���仯ѧʽΪ��TiO��x2x+�����������������Ϊ+n������������к���0.5n��Tiԭ�ӣ�
=1����Ti��Oԭ�Ӹ���֮��Ϊ1��1���仯ѧʽΪ��TiO��x2x+�����������������Ϊ+n������������к���0.5n��Tiԭ�ӣ�
(3)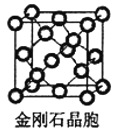 Ϊ���ʯ�����ṹ����������������ʯ���ƣ�ÿ��Alԭ����4��Nԭ����������ͬһ��Nԭ��������Alԭ�ӹ��ɵ����幹��Ϊ��������ṹ��ԭ�Ӿ����۷е�ϸߣ��þ����۵�ܸߣ�����Ϊԭ�Ӿ��壻
Ϊ���ʯ�����ṹ����������������ʯ���ƣ�ÿ��Alԭ����4��Nԭ����������ͬһ��Nԭ��������Alԭ�ӹ��ɵ����幹��Ϊ��������ṹ��ԭ�Ӿ����۷е�ϸߣ��þ����۵�ܸߣ�����Ϊԭ�Ӿ��壻
(4)�������ֻ�����������ܱ����룬����0.01mol��ɫ����ᄃ���ˮ��Һ�м��������������Һ������0.02molAgCl����������Clԭ���غ�֪��0.01mol��ɫ�����������������������ӣ�1���������ڽ磬��Ϊ����λ����5��������5��ˮ����λ���ڽ硢1��ˮ����Ϊ�ᾧˮ���ӣ������仯ѧʽΪ[TiCl(H2O)5]Cl2H2O��
(5)1����������γ���6���������ÿ�������2��������ӹ��ã���1mol����ľ�������3mol�������ԭ�������ֻ��3�����ӣ�����ԭ���γ�3�Թ��õ��Ӷԣ�ÿ��Oԭ���γ�2�����õ��Ӷԡ�ÿ��Hԭ���γ�1�����õ��Ӷԣ�ͼ����ʾ������Ѵ�8ԭ�ӽṹ��ԭ����OԪ�أ�H3BO3�������γ�3��B-O��������3��O-H���Լ���H3BO3������Bԭ�Ӹ����뼫�Լ�������Ϊ1��6��

����Ŀ��ijУѧϰС���ͬѧ���ʵ�飬�Ʊ���NH4��2Fe��SO4��26H2O��̽����ֽ���ɡ�ʵ�鲽�����£�
��.��ȡ7.0g��ҵ�����۷����ձ��У������ȵ�Na2CO3��Һϴ�ӣ���ˮϴ�������
��.��ȡ6.0g��������������ۼ���25mLijŨ�������м��ȣ����ȹ����в��ϲ�������ˮ������Ӧ��֡�
��.��ȴ�����˲�ϴ�ӹ��������ۣ������������۵�������
��.��������Һ�м���������NH4��2SO4���壬������������ȫ�ܽ⣬��һϵ�в����ø��﴿���ģ�NH4��2Fe��SO4��26H2O��
V.����NH4��2Fe��SO4��26H2O��ˮ�ã�NH4��2Fe��SO4��2���������ȷֽ�ʵ�顣
��֪�ڲ�ͬ�¶���FeSO47H2O���ܽ�������
�¶ȣ��棩 | 1 | 10 | 30 | 50 |
�ܽ�ȣ�g�� | 14.0 | 17.0 | 25.0 | 33.0 |
�ش��������⣺
��1����������ȵ�Na2CO3��Һϴ�ӹ�ҵ�����۵�Ŀ����__���������������۹�������Ϊ��__�����ȷ�Ӧ�������費�ϲ�������ˮ��Ŀ����__��
��2��������г�����Ӧ��ʣ�����۵���������Ϊ��__��
��3����NH4��2Fe��SO4��2�ֽ����̬���������N2��NH3��SO2��SO3��ˮ������������װ�ü��鲿�ֲ��

�ټ�����̬�����е�SO2��SO3ʱ��װ������˳������Ϊ__�������������ң���C��ʢ�ŵ��Լ�Ϊ__��
��װ��A��������__��
�ۼ����ַֽⲢ��ȴ��Ĵ������������������Ƿ��ж����������õ����Լ�Ϊ__��