��Ŀ����
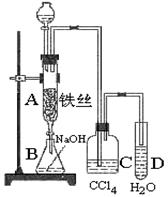
ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A(A�¶˻����ر�)�С�
(1)д��A�з�Ӧ�Ļ�ѧ����ʽ________________________________________________��
(2)�۲쵽A�е�������______________________________________________________��
(3)ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����____________________________________��д���йصĻ�ѧ����ʽ_____________________��
(4)C��ʢ��CCl4��������___________________��
(5)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���_____________________��������___________________��
������������Ҫ�����屽�Ʊ������е�ϸ�����⡣���ڱ���Һ��ķе�ϵͣ��ҷ�Ӧ![]() Ϊ���ȷ�Ӧ����A�й۲쵽������Ϊ����ӦҺ�У��к���ɫ�������A������HBr�л��е�Br2�ھ���CCl4ʱ�����գ����Ʊ����屽�г�����Br2��һ�����NaOH��Һ�У�������ӦBr2+2NaOH=NaBr+NaBrO+H2O����ȥ�����÷�ӦΪȡ����Ӧ�����HBr���ɣ���Ϊ�ӳɷ�Ӧ��û��HBr���ɣ���ֻ����D���Ƿ��д���H+��Br-���ɡ�
Ϊ���ȷ�Ӧ����A�й۲쵽������Ϊ����ӦҺ�У��к���ɫ�������A������HBr�л��е�Br2�ھ���CCl4ʱ�����գ����Ʊ����屽�г�����Br2��һ�����NaOH��Һ�У�������ӦBr2+2NaOH=NaBr+NaBrO+H2O����ȥ�����÷�ӦΪȡ����Ӧ�����HBr���ɣ���Ϊ�ӳɷ�Ӧ��û��HBr���ɣ���ֻ����D���Ƿ��д���H+��Br-���ɡ�
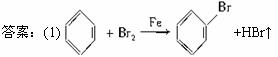
(2)��ӦҺ�У��к���ɫ�������A����
(3)��ȥ�����屽�е��� Br2+2NaOH![]() NaBr+NaBrO+H2O��
NaBr+NaBrO+H2O��
3Br2+6NaOH![]() 5NaBr+NaBrO3+3H2O
5NaBr+NaBrO3+3H2O
(4)��ȥ�廯�������е�������
(5)ʯ����Һ ��Һ���ɫ(�������������)

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�