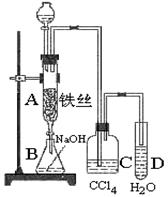
��Ŀ����
ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A(A�¶˻����ر�)�С�
(1)д��A�з�Ӧ�Ļ�ѧ����ʽ_________________________________��
(2)�۲쵽A�е�������______________________________________��
(3)ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����___________________��д���йصĻ�ѧ����ʽ________________��
(4)C��ʢ��CCl4��������______________________��
(5)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���_____________��������______________________________��
(1)C6H6+Br2![]() C6H5Br+HBr��
C6H5Br+HBr��
(2)��ӦҺ�У��к���ɫ�������A����
(3)��ȥ�����屽�е��� Br2+2NaOH![]() NaBr+NaBrO+H2O��3Br2+6NaOH
NaBr+NaBrO+H2O��3Br2+6NaOH![]() 5NaBr+ NaBrO3+3H2O
5NaBr+ NaBrO3+3H2O
(4)��ȥ�廯�������е�������
(5)ʯ����Һ����Һ���ɫ(���������)
�����������巴Ӧ�ų�����������ʹ��ӦҺ�У���ʹ��ӷ�����HBr�����л������������ʺ���ɫ��ͨ��CCl4ʱ���屻CCl4���գ��ʽ���D��Ϊ�ϴ���HBr���塣

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ