��Ŀ����
��16�֣���һ�������й�ʵ��������жϲ���ȷ���� ������ţ���ѡ�۷֣���
A������һ�����ʵ���Ũ����Һ������ʱƽ�ӿ̶��ߡ�
B���������Ȼ�̼���Ҵ������л��ܼ�����������ȡ��ˮ�е��塣
C����һ����ͨ�����壬�Ӵ�ֱ�ڹ��ߵķ�����Կ���һ�������ġ�ͨ·����
D������100mL 1mol/L ��NaOH��Һ�������4g ���������ƹ��塣
E���ò�˿պȡ������Һ����ɫ��Ӧ,û�й۲쵽��ɫ,���Ը���Һ�в�����Ԫ��
������ʵ������Ҫ480mL0.1mol/LNa2CO3��Һ������̼���ƾ������ƣ��ش���������
(1)Ӧ��������ƽ��ȡʮˮ��̼���ƣ�Na2CO3��10H2O������ g��
(2)���ڳ�����Ʒʱ,ҩƷ������ƽ������,���������ƽ�����ϣ�1g���������룬��ƽƽ��ʱ,��ʵ�ʳ�����ʮˮ��̼�������� (��ƫ�ߡ�ƫ�͡�����)��
(3) Na2CO3�� �� ��
(4) ��ʵ�����������,��Һ��Ũ�Ƚ��ᣨ��ƫ�ߡ�ƫ�͡�����)
A����ˮ����ʱ���ӿ̶���
B������ƿ�ڱڸ���ˮ���δ���ﴦ��
C���ߵ�ҡ�Ⱥ��ְ�Һ����ڿ̶����ּ�ˮ����
D��������̼���ƾ��岿��ʧ�ᾧˮ
(5)�������Ǽ�ˮ�����˿̶��ߣ���ȷ�Ĵ���������
(6)��������ѱ�ǩ�ϵ�����дһ��(����ͼ)��
A������һ�����ʵ���Ũ����Һ������ʱƽ�ӿ̶��ߡ�
B���������Ȼ�̼���Ҵ������л��ܼ�����������ȡ��ˮ�е��塣
C����һ����ͨ�����壬�Ӵ�ֱ�ڹ��ߵķ�����Կ���һ�������ġ�ͨ·����
D������100mL 1mol/L ��NaOH��Һ�������4g ���������ƹ��塣
E���ò�˿պȡ������Һ����ɫ��Ӧ,û�й۲쵽��ɫ,���Ը���Һ�в�����Ԫ��
������ʵ������Ҫ480mL0.1mol/LNa2CO3��Һ������̼���ƾ������ƣ��ش���������
(1)Ӧ��������ƽ��ȡʮˮ��̼���ƣ�Na2CO3��10H2O������ g��
(2)���ڳ�����Ʒʱ,ҩƷ������ƽ������,���������ƽ�����ϣ�1g���������룬��ƽƽ��ʱ,��ʵ�ʳ�����ʮˮ��̼�������� (��ƫ�ߡ�ƫ�͡�����)��
(3) Na2CO3�� �� ��
(4) ��ʵ�����������,��Һ��Ũ�Ƚ��ᣨ��ƫ�ߡ�ƫ�͡�����)
A����ˮ����ʱ���ӿ̶���
B������ƿ�ڱڸ���ˮ���δ���ﴦ��
C���ߵ�ҡ�Ⱥ��ְ�Һ����ڿ̶����ּ�ˮ����
D��������̼���ƾ��岿��ʧ�ᾧˮ
(5)�������Ǽ�ˮ�����˿̶��ߣ���ȷ�Ĵ���������
(6)��������ѱ�ǩ�ϵ�����дһ��(����ͼ)��

��һ��B D ������ţ���ѡ�۷֣���
������(1) 14.3g�� (2)ƫ�� (3) 500mL����ƿ����ͷ�ιܡ�
(4) A��ƫ�� B������ C��ƫ�� D��ƫ�� (5)��������
(6)
������(1) 14.3g�� (2)ƫ�� (3) 500mL����ƿ����ͷ�ιܡ�
(4) A��ƫ�� B������ C��ƫ�� D��ƫ�� (5)��������
(6)

�����������һ���Ҵ�������ˮ���ܣ����Բ�����������ȡ��������ƽ��������ʱ������ȡ��С�����һλ����˳�����NaOH������Ϊ4.0g������BD�Ǵ���ġ�
������(1)����ƿû��480mL�ģ������Ҫ��500mL������ƿ�����ƣ�����ҪNa2CO3��10H2O�����ʵ���Ϊ0.05mol������Ϊ14.3g��(2)������������ŷ���������Ĺ����������ʵ�ʵ�����С��(3)������Һʱ���õ���Ҫ������������ƽ��ҩ�ס��ձ�������������Ͳ��500mL����ƿ����ͷ�ιܡ�(4) ����c=
 ����ˮ����ʱ���ӿ̶��ߣ���Һ�����������ֵС������ƫ�ߡ�����ƿ�ڱڸ���ˮ���δ���ﴦ�����Ȳ�Ӱ�����ʵ����ʵ�����Ҳ��Ӱ����Һ�������������Ӱ�졣�ߵ�ҡ�Ⱥ��ְ�Һ����ڿ̶����ּ�ˮ���ϣ���Һ�����������ֵ������ƫ�͡�������̼���ƾ��岿��ʧ�ᾧˮ�����ʵ�����������ֵ������ƫ�ߡ�(5)�������ʱ��ˮ�����˿̶��ߣ�ֻ���������ƣ����ܰѶ�����������ȥ��
����ˮ����ʱ���ӿ̶��ߣ���Һ�����������ֵС������ƫ�ߡ�����ƿ�ڱڸ���ˮ���δ���ﴦ�����Ȳ�Ӱ�����ʵ����ʵ�����Ҳ��Ӱ����Һ�������������Ӱ�졣�ߵ�ҡ�Ⱥ��ְ�Һ����ڿ̶����ּ�ˮ���ϣ���Һ�����������ֵ������ƫ�͡�������̼���ƾ��岿��ʧ�ᾧˮ�����ʵ�����������ֵ������ƫ�ߡ�(5)�������ʱ��ˮ�����˿̶��ߣ�ֻ���������ƣ����ܰѶ�����������ȥ���������ڼ����������ʵ�����ʱ��Ҫע��������Һ������Ƿ�����Ӧ������ƿ�����û����ѡ����ӽ�������ƿ����������ƿ��������������ʵ�������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
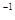
 ��ȫת��ΪBaSO4��������Ҫ��������Һ�����ʵ����ʵ���Ũ��֮��Ϊ�� ��
��ȫת��ΪBaSO4��������Ҫ��������Һ�����ʵ����ʵ���Ũ��֮��Ϊ�� ��