��Ŀ����
 ���أ�H2NCONH2�����������л����ʣ���Ҫ������[Fe��H2NCONH2��6]��NO3��3[�����������غ�������]��
���أ�H2NCONH2�����������л����ʣ���Ҫ������[Fe��H2NCONH2��6]��NO3��3[�����������غ�������]����1����̬Fe3+�ĺ�������Ų�ʽΪ
1s22s22p63s23p63d5
1s22s22p63s23p63d5
��C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳����N��O��C
N��O��C
����2�����ط�����C��Nԭ�ӵ��ӻ���ʽ�ֱ���
sp2��sp3
sp2��sp3
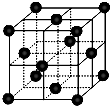
����3��[Fe��H2NCONH2��6]��NO3��3�С�H2NCONH2����Fe����֮�����������
���
���
����NO3-��Ϊ�ȵ������һ�ֻ�������SO3��
SO3��
��д��ѧʽ������4��CO2��NH3�ǹ�ҵ���Ʊ����ص���Ҫԭ�ϣ���̬CO2���ɱ����ľ����ṹ��ͼ��ʾ��
��1��CO2������Χ�Ⱦ����Ҿ��������CO2������
12
12
������ͭ��Ͻ�ľ����ṹ��ɱ����ƣ�������ΪAu������ΪCu����ͭ��Ͻ�����Au��Cuԭ����֮��Ϊ��
1��3
1��3
����������1��Feԭ�Ӻ��������Ϊ26��ԭ���γ��������Ȱ��ܲ�ߵ�ʧȥ���ӣ��ܲ�Խ�ߵĵ���Խ����ʧȥ��ͬһ�ܲ��а��ܼ��ߵ�ʧȥ���ӣ��ܼ�Խ��Խ����ʧȥ��
ͬ����������ҵ�һ�����ܳ��������ƣ�NԪ��ԭ�ӵ�2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬���������ͣ�ʧȥ������Ҫ�������ϸߣ���һ�����ܸ���ͬ��������Ԫ�أ�
��2�������صĽṹʽ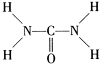 ��֪�����ط�����Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3���ݴ��жϣ�
��֪�����ط�����Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3���ݴ��жϣ�
��3��Fe�����пչ�������ط�����Nԭ�Ӻ��й¶Ե��ӣ������γ���λ����
ԭ��������ȣ��۵���������ȵ���Ϊ�ȵ����壬���ô�������д��
��4�����Զ���Ķ�����̼�����о�����֮����Ķ�����̼����λ�������ϣ�
�ڸ��ݾ�̯�����㾧���к���Au��Cuԭ����Ŀ���ݴ˼��㣮
ͬ����������ҵ�һ�����ܳ��������ƣ�NԪ��ԭ�ӵ�2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬���������ͣ�ʧȥ������Ҫ�������ϸߣ���һ�����ܸ���ͬ��������Ԫ�أ�
��2�������صĽṹʽ
 ��֪�����ط�����Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3���ݴ��жϣ�
��֪�����ط�����Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3���ݴ��жϣ���3��Fe�����пչ�������ط�����Nԭ�Ӻ��й¶Ե��ӣ������γ���λ����
ԭ��������ȣ��۵���������ȵ���Ϊ�ȵ����壬���ô�������д��
��4�����Զ���Ķ�����̼�����о�����֮����Ķ�����̼����λ�������ϣ�
�ڸ��ݾ�̯�����㾧���к���Au��Cuԭ����Ŀ���ݴ˼��㣮
����⣺��1��Feԭ�Ӻ�����26�����ӣ���������Ų�Ϊ1s22s22p63s23p63d64s2��Feԭ��ʧȥ4s�ܼ�2�����ӡ�3d�ܼ�1�������γ�Fe3+��Fe3+�����Ų�ʽΪ1s22s22p63s23p63d5��
ͬ����������ҵ�һ�����ܳ��������ƣ�NԪ��ԭ�ӵ�2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬���������ͣ�ʧȥ������Ҫ�������ϸߣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������N��O��C��
�ʴ�Ϊ��1s22s22p63s23p63d5��N��O��C��
��2�������ط��ӵĽṹʽ ��֪�����ط�����Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ʴ�Ϊ��sp2��sp3��
��֪�����ط�����Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ʴ�Ϊ��sp2��sp3��
��3��Fe�����пչ��������λ����ĿΪ6��Oԭ���ṩ�¶Ե��ӣ�H2NCONH2��Fe����֮���γ���λ����Nԭ������������Ϊ5����Nԭ�Ӽ�1����λ����ɣ������滻ΪSԭ�ӣ���SO3��NO3-��Ϊ�ȵ����壬
�ʴ�Ϊ����λ����SO3�ȣ�
��4�����Զ���Ķ�����̼�����о�����֮����Ķ�����̼����λ�������ϣ���������ȫ��֪����3��ÿ����4��������̼������֮�������֮����Ķ�����̼������4��3=12��
�ʴ�Ϊ��12��
��������ΪAu������ΪCu�������к���Auԭ����ĿΪ8��
=1�������к���Cuԭ����ĿΪ6��
=3����ͭ��Ͻ�����Au��Cuԭ����֮��Ϊ1��3��
�ʴ�Ϊ��1��3��
ͬ����������ҵ�һ�����ܳ��������ƣ�NԪ��ԭ�ӵ�2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬���������ͣ�ʧȥ������Ҫ�������ϸߣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������N��O��C��
�ʴ�Ϊ��1s22s22p63s23p63d5��N��O��C��
��2�������ط��ӵĽṹʽ
 ��֪�����ط�����Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ʴ�Ϊ��sp2��sp3��
��֪�����ط�����Cԭ�ӳ�2��C-N����1��C=O����û�й¶Ե��ӣ��ӻ������ĿΪ3��Cԭ�Ӳ�ȡsp2�ӻ���Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����ʴ�Ϊ��sp2��sp3����3��Fe�����пչ��������λ����ĿΪ6��Oԭ���ṩ�¶Ե��ӣ�H2NCONH2��Fe����֮���γ���λ����Nԭ������������Ϊ5����Nԭ�Ӽ�1����λ����ɣ������滻ΪSԭ�ӣ���SO3��NO3-��Ϊ�ȵ����壬
�ʴ�Ϊ����λ����SO3�ȣ�
��4�����Զ���Ķ�����̼�����о�����֮����Ķ�����̼����λ�������ϣ���������ȫ��֪����3��ÿ����4��������̼������֮�������֮����Ķ�����̼������4��3=12��
�ʴ�Ϊ��12��
��������ΪAu������ΪCu�������к���Auԭ����ĿΪ8��
| 1 |
| 8 |
| 1 |
| 2 |
�ʴ�Ϊ��1��3��
���������⿼���������Ų����ɡ��ӻ�������ۡ�������������ȣ��Ѷ��еȣ���3����ע��ȵ�����Ϊԭ��������ȣ��������������۵���������ȵ�����

��ϰ��ϵ�д�
 ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д�
�����Ŀ