��Ŀ����
����Ŀ��ij��ȤС���÷Ͼɶ�п��Ƥ�����������Ʊ���ˮ����п(ZnSO4��7H2O)

�����Ϣ���£�
�ٽ��������γ��������������pH����ͼA��ʾ��
��ZnSO4���ܽ��(������100gˮ���ܽ������)���¶ȱ仯������ͼB��ʾ��

��ش�
��1��Ϊ��߶�п��Ƥ�н������ӵĽ����ʣ����˿��ʵ����������Ũ�ȣ������Բ�ȡ�Ĵ�ʩ�У�_______(��дһ��)��
��2�����������������H2O2���������ӷ���ʽ��ʾH2O2������________��
��3��������е���pH��ΧΪ_______������pH��ѡ�õ��Լ�Ϊ_______��
A��ϡ���� B��������п C���������� D������п
��4�����鲽���������Һ���Ƿ���Fe3+�ɲ��õ�ʵ�鷽����_______��
��5���������Ҫ�õ��������в�����a����������Һ���־�Ĥ b����60�������ܼ� c����ȴ������ d����100�������ܼ� e������
�����������������ȷ˳��______�������ɸҸ�ʹ��)��
��6������V�У�ijͬѧ���ò�ͬ���·�ʽ������ȴ�ᶼ�����ZnSO4��7H2O������С�ֲ���ͼ��ʾ�����ݸ�ʵ������Ϊ�˵õ�������С��Ծ��Ľϴ�������ѡ��______��ʽ������ȴ�ᾧ��
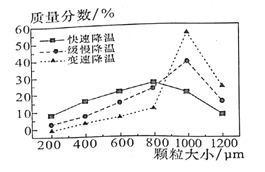
A�����ٽ��� B���������� C�����ٽ���
��7��ZnSO4�������Ʊ�����п����п��������Ƴɵĸ������Ե�أ��ܴ������ͨ���Ե�ض�50%�ĵ��ܣ���֪�õ�ص��ܷ�Ӧ��2K2FeO4+3Zn=Fe2O3+ZnO+2K2ZnO2���õ��������Ӧʽ��________��
���𰸡�����п��Ƥ�������Ȼ���� 2Fe2++H2O2+2H+=2Fe3++2H2O 3.8��pH<6.4 BD ȡ������Һ�������еμ�KSCN��Һ������Һ��Ϊ��ɫ����˵����Һ�к���Fe3+������ dabace C 2FeO42-+6e-+5H2O=Fe2O3+10OH-
��������
���������ͼ��֪����п��Ƥ�м���ϡ���ᣬп������ϡ���ᷴӦ��������п���������������˵õ���������п��������������Һ������Һ�м������˫��ˮ��˫��ˮ��������������Ϊ�����ӣ�����������п������п������ҺpHʹ������ת��Ϊ��������ȥ�����˵õ���������п����Һ����ͼB��֪�����������¶ȡ���ȴ�ᾧ�õ�������п���壻������п����ͨ���ؽᾧ���������ˮ����п���塣
��1��Ϊ��߶�п��Ƥ�н������ӵĽ����ʣ����˿��ʵ����������Ũ�ȣ������Բ�ȡ�������߷�Ӧ�¶ȡ������п��Ƥ�ͽ���ȴ�ʩ���ʴ�Ϊ������п��Ƥ�������Ȼ���裻
��2���������м������H2O2��Ŀ���ǽ�������������Ϊ�����ӣ���Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O���ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��3����ͼA��֪���������м���������п������п������Һ3.8��pH<6.4��ʹFe3+��ȫ�������ֲ�ʹZn2+�������ʴ�Ϊ��3.8��pH<6.4��BD��
��4��Fe3+��KSCN��Һ��Ӧ����Ѫ��ɫ�����軯����Һ�����鲽����������Һ���Ƿ���Fe3+Ӧѡ��KSCN��Һ����������Ϊȡ������Һ�������еμ�KSCN��Һ������Һ��Ϊ��ɫ����˵����Һ�к���Fe3+���������ʴ�Ϊ��ȡ������Һ�������еμ�KSCN��Һ������Һ��Ϊ��ɫ����˵����Һ�к���Fe3+��������
��5����ͼB��֪��Ҫ����Һ�еõ��ֲ�Ʒ�����ȼ���������ֱ�����־�Ĥ���ﵽ����״̬����ȥ�����ܼ�����60��ʱ��Ʒ���ܽ����ʽ�����60���������ܼ��������־�Ĥ���ﵽ����״̬��������ȴ�����£�ʹ��Ʒ�����������ˣ�ʹ��Һ���룬�õ��ֲ�Ʒ���ʲ�������ȷ˳��Ϊdabace���ʴ�Ϊ��dabace��
��6�����ͼ���֪�ڱ��ٽ��µ�����µõ��Ŀ����ϴ�ѡC���ʴ�Ϊ��C��
��7���ɵ���ܷ�Ӧ����ʽ��֪����������������Ϸŵ緢����ԭ��Ӧ����Fe2O3���缫��ӦʽΪ2FeO42-+6e-+5H2O=Fe2O3+10OH-���ʴ�Ϊ��2FeO42-+6e-+5H2O=Fe2O3+10OH-��



