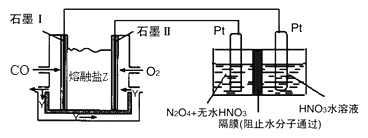
��Ŀ����
����Ŀ���������ǵ��������ı����������������и�ǿ�������ԡ�ʵ���ҿɽ�����ͨ����ѹ�ŵ������ȡ������3O2�ŵ�,2O3��
(1)����������Ӧ����30%������ת��Ϊ���������û������ƽ��Ħ������Ϊ________g��mol��1(����һλС��)��
(2)��8 L����ͨ���ŵ�ָܺ���ԭ״�����õ�����6.5 L�����г���Ϊ________L��
(3)ʵ���ҽ������ͳ����Ļ������0.896 L(��״��)ͨ��ʢ��20.0 gͭ�۵ķ�Ӧ�����У���ּ��Ⱥ�ĩ��������Ϊ21.6 g����ԭ������г������������Ϊ���٣�(д���������)___________
���𰸡� 35.6 3 ���������£�O3��O2���ܺ�ͭ�۷�Ӧ���ʷ�ĩ���ӵ�������ΪO2��O3�������������������к���O2 x mol������O3 y mol������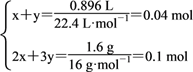 ���
���![]() ���Ի�������г������������Ϊ50%��
���Ի�������г������������Ϊ50%��
��������(1)����1 mol O2��
3O2��![]() ��2O3��������n
��2O3��������n
3 mol��������2 mol����1 mol
0��3 mol��������������0.1 mol
���ݣ�![]() ��
��![]() ��
��![]() ��35.6 g��mol��1��
��35.6 g��mol��1��
(2) 3O2����![]() ����2O3��������������V
����2O3��������������V
��3�������������2�������������1���
������������ ��V(L)��������8��6.5��1.5 L
��V��3 L��
(3)���������£�O3��O2���ܺ�ͭ�۷�Ӧ���ʷ�ĩ���ӵ�������ΪO2��O3�������������������к���O2 x mol������O3 y mol��
����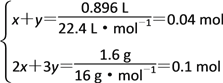
���![]()
���Ի�������г������������Ϊ50%��

 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д� �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�