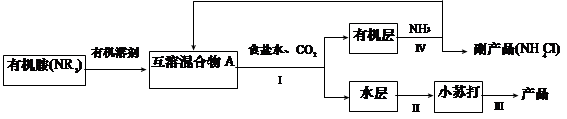��Ŀ����
�ܣ�Co������������һ����Ҫ�Ļ���ԭ�ϣ���ҵ������CoCO3+O2��CoxOy+ CO2��Ӧ��������Ӧ���ܵ������ʵ�����п���������װ������ȡ�ܵ������ﲢ�ⶨ�������ɡ�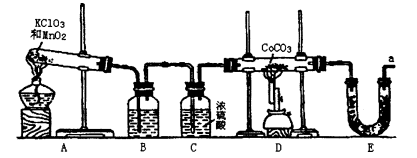
����д���пհף�
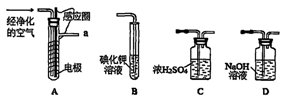
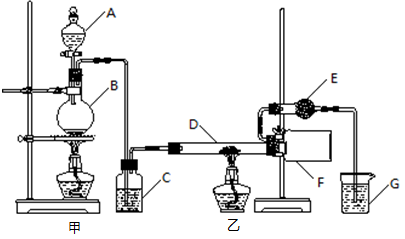
��1��д��Aװ�õĴ��Թ������Ӧ�Ļ�ѧ����ʽ ��
��2��Eװ�õ�U�ι���ʢ�ŵ������� ��
A��P2O5 B����ˮCaCl2 C����ʯ�� D����ˮCuSO4
��3��O3�������Ա�O2ǿ����֪�Ƶõ�O2�к���������Cl2��O3����Bװ������ʢ�ŵ�������
A��NaOH��Һ B������NaHCO3��Һ C������NaCI��Һ D��KI��Һ
��4��ʵ�����ʱ�����ȳ�ȥAװ���еľƾ��ƣ������� ��
��5����CoCO3��ȫת��ΪCoxOy�����Ƶ�E������4.40g��D���ڲ������ʵ�������8.30g��������CoxOy�Ļ�ѧʽΪ ��
��6����ʵ��װ�ô���һ���Ƚϴ��ȱ�ݣ�������� ��
��1�� 2KClO3 2KCl+3O2��
2KCl+3O2��
��2��C ��2�֣� ��3��D ��2�֣� ��4�� ������2�֣���ϸд���֣�
��5��Co2O3��3�֣� ��6����a����һװ�м�ʯ�ҵĸ���ܣ�2�֣����������������CO2�����֣�
���������������װ��ͼ��֪��ʵ��ͨ���ⶨװ��E�е����أ�ȷ�����ɵĶ�����̼��������������n(C)��n(Co)��֪CoxOy��Co�����ʵ�������������Co��������װ��D���ڲ���������CoxOy���������Ԫ���������ټ�����ԭ�ӵ����ʵ���������ԭ�����ʵ���֮��ȷ����ѧʽ��
��1���ڶ������������������������£�����طֽ������Ȼ��ء���������Ӧ�Ļ�ѧ����ʽΪ2KClO3 2KCl+3O2����
2KCl+3O2����
��2��Eװ�õ�U�ι���ʢ�ŵ�������������װ��D���ɵĶ�����̼��A��P2O5��B����ˮCaCl2��D����ˮCuSO4���������ն�����̼��C����ʯ���Ǽ��Ը�������������ն�����̼����װ��E���Լ�Ϊ��ʯ�ң����Դ�ѡC��
��3���Ƶõ�O2�к���������Cl2��O3��Bװ������ʢ�ŵ�������������Cl2�ͳ�����A��NaOH��Һ�����������������������ճ���������A����ȷ��B������NaHCO3��Һ�����������������ɶ�����̼��Ӱ�������̼�����IJⶨ���Ҳ������ճ�������B�����ϣ�C������NaCl��Һ�������������ͳ�������C����D��KI��Һ���������ͳ���������D���ϣ���ѡD��
��4��ʵ�����ʱ�����ȳ�ȥAװ���еľƾ��ƣ��ᵼ��װ��A�е�ѹǿ���ͣ�������������
��5��E������4.40g�Ƕ�����̼�����������ʵ���Ϊ4.40g��44g/mol��0.1mol�����ݻ�ѧʽCoCO3��֪n(Co)��n(C)��0.1mol����Co������Ϊ0.1mol��59g/mol��5.9g��D���ڲ������ʵ�����8.30g��CoxOy��������CoxOy����Ԫ������Ϊ8.30g��5.9g��2.4g����ԭ�ӵ����ʵ���n(O)��2.4g��16/mol��0.15mol������n(Co):n(C)��0.1mol:0.15mol��2:3�������ܵ�������Ļ�ѧʽΪCo2O3��
��6������װ��E�м�ʯ��Ҳ�������տ�����ˮ������������̼��Ӱ�������̼�����IJⶨ�����Ӧ��a����һװ�м�ʯ�ҵĸ���ܣ����տ�����ˮ������������̼����ֹ����װ��E�С�
���㣺����ʵ�鷽�������װ�õ����⡢�����ᴿ����ѧ�����

 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�ijʵ��С����������װ��(���̶ֹ�װ����)�Ʊ�������(Ca3N2)����̽����ʵ��ʽ��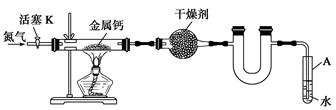
��1����Ӧ������ĩ�˵��ܱ���ʼ�ղ����Թ�A��ˮ�У�Ŀ����_____________________��
��2���Ʊ������ƵIJ��������ǣ�
�ٴ���K��ͨ��N2��
�ڵ�ȼ�ƾ��ƣ����з�Ӧ��
�۷�Ӧ������__________________________________________________________��
�ܲ��װ�ã�ȡ�����
��3�����ݼ�¼���£������跴Ӧ��ȫ��
| ��Ӳ�ʹ��� ��m0/g | Ӳ�ʹ���Ƶ� ����m1/g | Ӳ�ʹ������ ������m2/g |
| 14.80 | 15.08 | 15.15 |
�� ����õ�ʵ��ʽCaxN2������x��________��
����ͨ���N2�л�������O2����Ƚ�x��3�Ĵ�С���������ж����ݣ�
______________________________________________________________________��
����ʵ��������ܴﵽ���ӦĿ�ĵ���
| ��� | ʵ������ | ʵ��Ŀ�� |
| A�� | ��ʢ��10��0��1 mol/L AgNO3��Һ���Թ��еμ�0��1 mol/L NaCl��Һ���������г������ɣ��������еμ�0��1 mol/L Na2S��Һ | ֤��AgCl������ת��Ϊ�ܽ�ȸ�С��Ag2S���� |
| B�� | ��2 mL�ױ��м���3��KMnO4������Һ������2mL�����м���3��KMnO4������Һ���� | ֤���뱽�������ļ��ױ����� |
| C�� | ��Na2SiO3��Һ��ͨ��CO2 | ֤��̼������Աȹ���ǿ |
| D�� | �ڵ�����Һ�м���ϡ���ᣬˮԡ���ȣ�һ��ʱ����ټ������Ƶ�������ͭ������ | ��֤������ˮ�� |

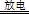 2O3����
2O3����