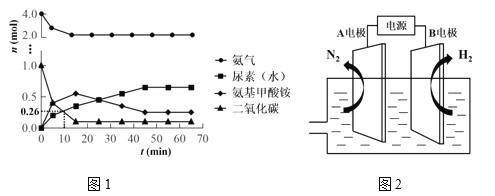
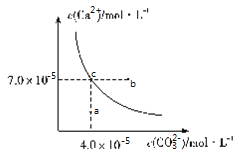
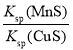��Ŀ����
����Ŀ����֪![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������
����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������![]() ��
��![]() ��
��![]() ��
��![]() �����ڱ������ڣ�
�����ڱ������ڣ�![]() ԭ�Ӻ���������δ�ɶԵ��ӣ�
ԭ�Ӻ���������δ�ɶԵ��ӣ�![]() �ĵ�һ�����ܱ�ͬ������������Ԫ�صĶ���
�ĵ�һ�����ܱ�ͬ������������Ԫ�صĶ���![]() ԭ����ͬ����Ԫ��ԭ���а뾶���ϡ������ԭ�ӳ��⣩��
ԭ����ͬ����Ԫ��ԭ���а뾶���ϡ������ԭ�ӳ��⣩��![]() ��
��![]() λ�ڲ�ͬ���ڣ�
λ�ڲ�ͬ���ڣ�![]() ԭ�Ӻ���������������
ԭ�Ӻ���������������![]() ��ͬ����������Ӳ���������ӡ������������Ϣ���ش��������⡣
��ͬ����������Ӳ���������ӡ������������Ϣ���ش��������⡣
��1��![]() ��
��![]() ��
��![]() ��
��![]() ����Ԫ�صĵ縺���ɴ�С������˳��Ϊ_________��
����Ԫ�صĵ縺���ɴ�С������˳��Ϊ_________��
��2��![]() ���⻯��ĽṹʽΪ_________������ӵĿռ乹��Ϊ________��
���⻯��ĽṹʽΪ_________������ӵĿռ乹��Ϊ________��
��3��![]() ��ij�ֻ�����Ľṹ��ͼ��ʾ����֪���������ð�����ѧ���ͷ��Ӽ�����������˻������и������Ӽ�����������___________��
��ij�ֻ�����Ľṹ��ͼ��ʾ����֪���������ð�����ѧ���ͷ��Ӽ�����������˻������и������Ӽ�����������___________��
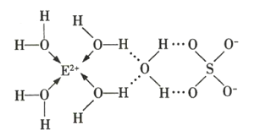
��4��![]() ��
��![]() ����̬�⻯����ȣ�_________���ѧʽ����ͬ���ķе���ߣ�
����̬�⻯����ȣ�_________���ѧʽ����ͬ���ķе���ߣ�![]() ��
��![]() ����̬�⻯����ȣ�________�ķе���ߡ�
����̬�⻯����ȣ�________�ķе���ߡ�
��5��![]() ���ȶ��������У�����ԭ�Ӳ�ȡ________�ӻ�������������ӵĿռ乹��Ϊ______��
���ȶ��������У�����ԭ�Ӳ�ȡ________�ӻ�������������ӵĿռ乹��Ϊ______��
���𰸡�![]()
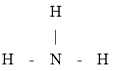 ������ ���Ӽ������ۼ�����λ�������
������ ���Ӽ������ۼ�����λ������� ![]()
![]() sp ֱ����
sp ֱ����
��������
�������֪��A��B��D�����λ��Ϊ ����B�ĵ�һ�����ܱ�ͬ������������Ԫ�صĶ���Aԭ�Ӻ���������δ�ɶԵ��ӣ���B��
����B�ĵ�һ�����ܱ�ͬ������������Ԫ�صĶ���Aԭ�Ӻ���������δ�ɶԵ��ӣ���B��![]() ������ڰ����״̬������֪BΪN��AΪC��DΪ
������ڰ����״̬������֪BΪN��AΪC��DΪ![]() ����Cԭ����ͬ����Ԫ��ԭ���а뾶���ϡ������ԭ�ӳ��⣩����֪CΪ
����Cԭ����ͬ����Ԫ��ԭ���а뾶���ϡ������ԭ�ӳ��⣩����֪CΪ![]() ��
��![]() Ϊ�������ڵ�Ԫ���������1�����ӣ���������Ӳ���������ӣ���
Ϊ�������ڵ�Ԫ���������1�����ӣ���������Ӳ���������ӣ���![]() �Ļ�̬ԭ�ӵĵ����Ų�ʽΪ
�Ļ�̬ԭ�ӵĵ����Ų�ʽΪ![]() ��
��![]() Ϊ
Ϊ![]() ��
��
��1��A��B��C��D�ֱ�Ϊ![]() ��
��![]() �����ݵ縺�Եĵݱ���ɿ�֪���縺�ԣ�
�����ݵ縺�Եĵݱ���ɿ�֪���縺�ԣ�![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��2��B���⻯��Ϊ![]() ������ԭ��
������ԭ��![]() ��ȡ
��ȡ![]() �ӻ������ӵĿռ乹��Ϊ�����Σ��ʴ�Ϊ��
�ӻ������ӵĿռ乹��Ϊ�����Σ��ʴ�Ϊ��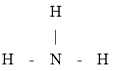 �������Σ�
��������
��3������ͼʾ���жϳ�![]() ���Ӻ�
���Ӻ�![]() �������λ����ͬʱˮ���Ӽ仹���������
�������λ����ͬʱˮ���Ӽ仹���������![]() �����ڴ��ڹ��ۼ����û������к��������ӣ����������Ӽ����ʴ�Ϊ�����Ӽ������ۼ�����λ���������
�����ڴ��ڹ��ۼ����û������к��������ӣ����������Ӽ����ʴ�Ϊ�����Ӽ������ۼ�����λ���������
��4��A��B����̬�⻯��ֱ�Ϊ![]() ��
��![]() ����
����![]() ���Ӽ����������ʷе㣺
���Ӽ����������ʷе㣺![]() ��A��D����̬�⻯��ֱ�Ϊ
��A��D����̬�⻯��ֱ�Ϊ![]() ��
��![]() ��������ṹ���ƣ�
��������ṹ���ƣ�![]() ����Է�����������
����Է�����������![]() �����Էе㣺
�����Էе㣺![]() ���ʴ�Ϊ��NH3��SiH4��
���ʴ�Ϊ��NH3��SiH4��
��5��![]() ��
��![]() ԭ�Ӳ�ȡsp�ӻ���
ԭ�Ӳ�ȡsp�ӻ���![]() ���ӳ�ֱ���Σ��ʴ�Ϊ��sp��ֱ���Ρ�
���ӳ�ֱ���Σ��ʴ�Ϊ��sp��ֱ���Ρ�

 ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�