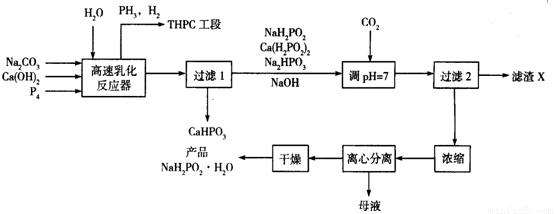
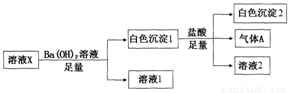
��Ŀ����
�Ȼ�李��״���������������Ҫ�����
��1����֪��
I NH4Cl(s)=NH3(g)+HCl(g) ��H=��l63.9 kJ��mol-1
II HCl(g)+CH3OH(g) CH3Cl(g)+H2O(g) ��H=-31.9kJ��mol-1
CH3Cl(g)+H2O(g) ��H=-31.9kJ��mol-1
III NH4Cl(s)+CH3OH(g) NH3(g)+CH3Cl(g)+H2O(g)
NH3(g)+CH3Cl(g)+H2O(g)
�� ��ӦIII��_________���������Է���Ӧ����ϸ��¶ȡ������ϵ��¶ȡ����κ��¶ȡ�����������_______________��
�� ͼ1�Ƿ�ӦIIIʹ�����ֲ�ͬ����ʱ�õ���CH3Cl�������¶ȹ�ϵ�ı仯ͼ��
��֪����������������������n(�״�)��n(�Ȼ��)��ֵ���״������ٶȡ���Ӧʱ��Ȳ�����������ͬ��
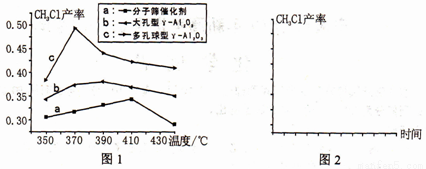
ͼ1 ��a����CH3Cl������������С��ԭ����___________������ͼ2�л���������������ͬʱ����370 ����ʹ�����ֲ�ͬ��������Ӧƽ��ʱ��CH3Cl�IJ�����ʱ���ϵ�ı仯���ߣ�����a��b��c�����Ӧ�����ߡ�_______________
��2��25 ��ʱ����ijŨ�ȵ�NH4Cl��Һ�еμ�һ�����İ�ˮ�����ԣ���ʱ�����Һ��c(Cl-)= 0.36mol �� L-1��������Һ��c (NH3 �� H2O)��_______mol �� L-1����25��ʱ��NH3��H2O��Kb=1.8��10-5��
��3�����Al2O3��Ĥ����Ϊ�������塢ģ��ϳ����ײ��ϵ���;�����Ըߴ���Ƭ��Ϊ�������������Ϊ������һ���ܶȵ�������Һ��Ϊ����ʽ��е�⣬���ɳ�����ȡ���Al2O3Ĥ����д������ȡ���̵������缫��Ӧ��__________________��
 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�


 ��Ϊͬ���칹��
��Ϊͬ���칹��