��Ŀ����
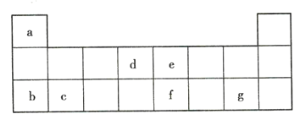
����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��û�ѧ����ش��������⣺
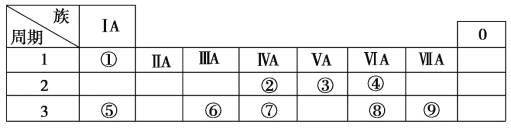
(1)����Ԫ�آߵ�ԭ�ӽṹʾ��ͼ_____________��
(2)�ڡ��ۡ��ߵ���ۺ������������ǿ������˳����_______________(�ѧʽ)��
(3)�١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ����д������һ�ֻ�����Ļ�ѧʽ��_______________��
(4)д��Ԫ�آ��γɵĹ�������ĵ���ʽ__________________��
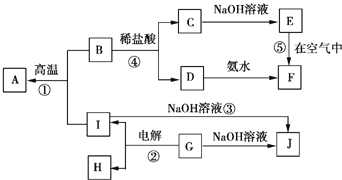
(5)�ɱ���Ԫ���γɵij������� X��Y��Z��M��N �ɷ������·�Ӧ��
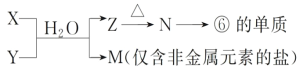
X ��Һ�� Y ��Һ��Ӧ�����ӷ���ʽΪ_____��
���𰸡�![]() HNO3>H2CO3>H2SiO3 NaOH
HNO3>H2CO3>H2SiO3 NaOH ![]() Al3++3NH3��H2O===Al(OH)3��+3NH4+
Al3++3NH3��H2O===Al(OH)3��+3NH4+
��������
��Ԫ�����ڱ���֪������H������C������N������O������Na������Al������Si������S������Cl���ݴ˷����ɽ�����⡣
(1)������������������Si����ԭ�ӽṹʾ��ͼΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2) �ڡ��ۡ��߷ֱ�ΪC��N��Si��Ԫ�طǽ����ԣ�N>C>Si���ǽ�����Խǿ��������������Ӧˮ��������Ծ�Խǿ������������������Ӧˮ��������ԣ�HNO3>H2CO3>H2SiO3���ʴ�Ϊ��HNO3>H2CO3>H2SiO3��
(3) �١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ����������NaOH���ʴ�Ϊ��NaOH��
(4)Ԫ�آ��γɵĹ�������ΪNa2O2�������ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5)��֪��Ԫ����Al����Z��N�ķ�Ӧ�����Ʋ�NΪAl2O3����ZΪAl(OH)3������Ϊ�����Ϣ˵��M�ǽ����зǽ������Σ�MΪ��Σ������Ʋ�XΪ���Σ�YΪ����������X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪAl3++3NH3��H2O===Al(OH)3��+3NH4+���ʴ�Ϊ��Al3++3NH3��H2O===Al(OH)3��+3NH4+��

 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�����Ŀ��ijС��ͨ��ʵ���о�Na2O2��ˮ�ķ�Ӧ��
���� | ���� |
��ʢ��4gNa2O2���ձ��м���50mL����ˮ�õ���Һa | ���з�Ӧ��������ʹ������ľ����ȼ������ |
ȡ5mL��Һa���Թ��У��������η�̪ | ��.��Һ��� ��.10���ֺ���Һ��ɫ���Ա�dz���Ժ���Һ��Ϊ��ɫ |
��1��Na2O2��ˮ��Ӧ�Ļ�ѧ����ʽ___��
��2��������Һ��ɫ��������Һa�д��ڽ϶��H2O2���̪�����˷�Ӧ��
��.��ͬѧͨ��ʵ��֤ʵ��H2O2�Ĵ��ڣ�ȡ������Һa�������Լ�___(�ѧʽ)�������������
��.��ͬѧ�������ϻ�Ϥ����KMnO4���ԲⶨH2O2�ĺ�����ȡ15.00mL��Һa����ϡH2SO4�ữ����μ���0.003molL-1KMnO4��Һ���������壬��Һ��ɫ���ʿ�ʼ�������죬���յ�ʱ������20.00mLKMnO4��Һ��
����ƽ��___MnO4-+___H2O2+___=___Mn2++___O2��+___H2O
����Һa��c(H2O2)=___molL-1��
����Һ��ɫ���ʿ�ʼ���������ԭ�������___��
��3��Ϊ̽����������ԭ��ͬѧ�Ǽ�������������ʵ�飺
��.��H2O2��Һ�е������η�̪��������5��0.1molL-1NaOH��Һ����Һ�����Ѹ�ٱ���ɫ�Ҳ������壬10���Ӻ���Һ����ɫ��
��.��0.1molL-1NaOH��Һ�е������η�̪�ģ�����Һ��죬10���Ӻ���Һ��ɫ�����Ա仯�������Һ��ͨ��O2����Һ��ɫ�����Ա仯��
�ٴ�ʵ���͢��У��ɵó��Ľ�����___��
��ͬѧ�ǽ�һ��ͨ��ʵ��֤ʵ����Һa�е����̪��H2O2���̪�����˻�ѧ��Ӧ��ʵ�鷽���ǣ�ȡ������Һa���Թ��У�___��