��Ŀ����
ʵ���ó�����0.1mol/LijһԪ�ᣨHA����Һ��pH������l��0.1mol/LijһԪ�BOH����Һ�c��H+��/c��OH-��=10-12������������Һ�������Ϻ�������Һ������ӵ����ʵ���Ũ�ȵĹ�ϵ��ȷ���ǣ�������
| A��c��B+����c��A-����c��OH-����c��H+�� | B��c��A-����c��B+����c��H+����c��OH-�� |
| C��c��H+����c��A-����c��OH-����c��B+�� | D��c��B+����c��A-����c��H+����c��OH-�� |
������0.1mol/LijһԪ�ᣨHA����Һ��pH������l��˵��HAû����ȫ���룬Ϊ���ᣬ0.1mol/LijһԪ�BOH����Һ��
=10-12�����c��H+����c��OH-��=10-14����֪c��OH-��=0.1mol/L����BOHΪǿ����߷�Ӧ����ǿ�������Σ���Һ�ʼ��ԣ���c��OH-����c��H+��������A-����ˮ������HA����c��B+����c��A-�����ε�ˮ��̶�һ�������Ӧ��c��A-����c��OH-������c��B+����c��A-����c��OH-����c��H+����
��ѡA��
| c(H+) |
| c(OH-) |
��ѡA��

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
�����Ŀ
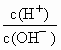 ��10��12������������Һ�������Ϻ�������Һ������ӵ����ʵ���Ũ���ɴ�С˳����
��10��12������������Һ�������Ϻ�������Һ������ӵ����ʵ���Ũ���ɴ�С˳����