��Ŀ����
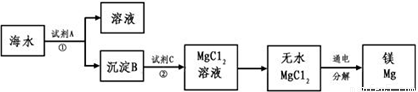
1808�꣬Ӣ����ѧ�Ҵ�ά�üػ�ԭ����þ�������Ƶ�������þ��þ�Ǻ��չ�ҵ����Ҫ���ϣ�þ�Ͻ���������ɻ�����������������ȣ�һ�ܳ����ٷɻ�Լ��5%��þ�Ͻ���һö����һ������100��200����þ�Ͻ�þ��Ϊһ��ǿ��ԭ�����������ѡ�ﯡ�����˵ȵ������У�þ��ȼ�յ�������������ȱ�ٵ�����þ���ǽ����̻������ԭ�ϣ�þ����Ͻ���һ����;�ܹ㷺�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ģ��Ӻ�ˮ����Ҫ��NaCl��MgSO4������ȡ����þ����Ҫ������ͼ��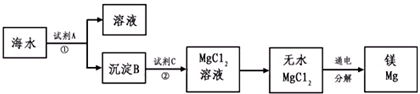
�ش��������⣺
��1��Mg�����ڱ��е�λ��
��2������ٵ����ӷ���ʽ
��3���õ���ʽ��ʾ�Ȼ�þ���γɹ���
��4����ˮMgCl2������״̬�£�ͨ�������Mg��Cl2���÷�Ӧ�Ļ�ѧ����ʽΪ��
��5����ά��þ�Ļ�ѧ����ʽΪ
��6����ƽ���з�Ӧ�ķ���ʽ��
��UF4+Mg--U+MgF2
��Mg+HNO3--Mg��NO3��2+N2O��+H2O��
��������ˮ�к���þ���ӣ���ˮ�м���Ca��OH��2����Mg��OH��2������������Ӧ2OH-+Mg2+=Mg��OH��2������Mg��OH��2����ϡ����õ�MgCl2��������ӦMg��OH��2+2H+=Mg2++2H2O����MgCl2��Һ��HCl��Χ�����ɵõ�MgCl2���壬�������MgCl2�õ�Mg����MgCl2�����ڣ�
Mg+Cl2����
| ||
����⣺��ˮ�к���þ���ӣ���ˮ�м���Ca��OH��2����Mg��OH��2������������Ӧ2OH-+Mg2+=Mg��OH��2������Mg��OH��2����ϡ����õ�MgCl2��������ӦMg��OH��2+2H+=Mg2++2H2O����MgCl2��Һ��HCl��Χ�����ɵõ�MgCl2���壬�������MgCl2�õ�Mg����MgCl2�����ڣ�
Mg+Cl2����
��1��Mgԭ�Ӻ�����3�����Ӳ㡢�����ĵ�������2����Mgλ�ڵ������ڵ�IIA�壬ͨ�����Ϸ���֪��A��Ca��OH��2��Һ��C�����ᣬ
�ʴ�Ϊ���������ڵڢ�A�壻Ca��OH��2��Һ�����
��2��ͨ�����Ϸ���֪���÷�Ӧ���ӷ���ʽΪ��2OH-+Mg2+=Mg��OH��2�����ʴ�Ϊ��2OH-+Mg2+=Mg��OH��2����
��3���Ȼ�þΪ���ӻ�����������γ����Ӽ������γɹ���Ϊ ��
��
�ʴ�Ϊ�� ��
��
��4����������Ȼ�þ����þ����������ⷴӦ����ʽΪ��MgCl2�����ڣ�
Mg+Cl2�����ʴ�Ϊ��MgCl2�����ڣ�
Mg+Cl2����
��5���ػ�ԭ����þ�õ������غ�þ����Ӧ����ʽΪ2K+MgO�TK2O+Mg���ʴ�Ϊ��2K+MgO�TK2O+Mg��
��6����UF4+Mg--U+MgF2��MgԪ�ػ��ϼ���0�۱�Ϊ+2�ۡ�UԪ�ػ��ϼ���+4�۱�Ϊ0�ۣ����ʧ������С��������4����÷�Ӧ����ʽΪ��UF4+2Mg=U+2MgF2��
�ʴ�Ϊ��1��2��1��2��
��Mg+HNO3--Mg��NO3��2+N2O��+H2O��MgԪ�ػ��ϼ���0�۱�Ϊ+2�ۡ�NԪ�ػ��ϼ���+5�۱�Ϊ+1�ۣ����ʧ������С��������8���ٽ��ԭ���غ���ƽ����ʽ���÷�Ӧ����ʽΪ��4Mg+10HNO3=4Mg��NO3��2+N2O��+5H2O��
�ʴ�Ϊ��4��10��4��1��5��
| ||
��1��Mgԭ�Ӻ�����3�����Ӳ㡢�����ĵ�������2����Mgλ�ڵ������ڵ�IIA�壬ͨ�����Ϸ���֪��A��Ca��OH��2��Һ��C�����ᣬ
�ʴ�Ϊ���������ڵڢ�A�壻Ca��OH��2��Һ�����
��2��ͨ�����Ϸ���֪���÷�Ӧ���ӷ���ʽΪ��2OH-+Mg2+=Mg��OH��2�����ʴ�Ϊ��2OH-+Mg2+=Mg��OH��2����
��3���Ȼ�þΪ���ӻ�����������γ����Ӽ������γɹ���Ϊ
 ��
���ʴ�Ϊ��
 ��
����4����������Ȼ�þ����þ����������ⷴӦ����ʽΪ��MgCl2�����ڣ�
| ||
| ||
��5���ػ�ԭ����þ�õ������غ�þ����Ӧ����ʽΪ2K+MgO�TK2O+Mg���ʴ�Ϊ��2K+MgO�TK2O+Mg��
��6����UF4+Mg--U+MgF2��MgԪ�ػ��ϼ���0�۱�Ϊ+2�ۡ�UԪ�ػ��ϼ���+4�۱�Ϊ0�ۣ����ʧ������С��������4����÷�Ӧ����ʽΪ��UF4+2Mg=U+2MgF2��
�ʴ�Ϊ��1��2��1��2��
��Mg+HNO3--Mg��NO3��2+N2O��+H2O��MgԪ�ػ��ϼ���0�۱�Ϊ+2�ۡ�NԪ�ػ��ϼ���+5�۱�Ϊ+1�ۣ����ʧ������С��������8���ٽ��ԭ���غ���ƽ����ʽ���÷�Ӧ����ʽΪ��4Mg+10HNO3=4Mg��NO3��2+N2O��+5H2O��
�ʴ�Ϊ��4��10��4��1��5��
���������⿼���˺�ˮ��þ����ȡ���漰������ԭ��Ӧ����ƽ������ʽ����д��֪ʶ�㣬֪������ͼ�з����ķ�Ӧ�����뷽�����ѵ��ǵ���ʽ��ʾ�������γɹ��̵���д����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
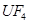 +
+ 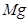 ����
����
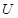 +
+ 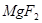
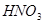 ����
����
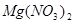 +
+ 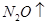 +
+
