��Ŀ����
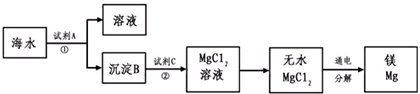
1808�꣬Ӣ����ѧ�Ҵ�ά�üػ�ԭ����þ�������Ƶ�������þ��þ�Ǻ��չ�ҵ����Ҫ���ϣ�þ�Ͻ���������ɻ�����������������ȣ�һ�ܳ����ٷɻ�Լ��5%��þ�Ͻ���һö����һ������100��200����þ�Ͻ�þ��Ϊһ��ǿ��ԭ�����������ѡ�ﯡ�����˵ȵ������У�þ��ȼ�յ�������������ȱ�ٵ�����þ���ǽ����̻������ԭ�ϡ�þ����Ͻ���һ����;�ܹ㷺�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ��Ӻ�ˮ����Ҫ��NaCl��MgSO4������ȡ����þ����Ҫ�������£�
�ش��������⣺
(1)Mg�����ڱ��е�λ��______________________���Լ�A����ѡ��_____________________���Լ�C��ѡ��_______________________��
(2)����ٵ����ӷ���ʽ_________________________________________��
(3)�õ���ʽ��ʾ�Ȼ�þ���γɹ���_______________________________��
(4)��ˮMgCl2������״̬�£�ͨ�������Mg��Cl2,�÷�Ӧ�Ļ�ѧ����ʽΪ��_____________________________________________________��
(5)��ά��þ�Ļ�ѧ����ʽΪ_____________________________________��
(6)��ƽ���з�Ӧ�ķ���ʽ��
�� 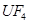 +
+ 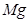 ����
����
 +
+ 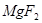
�� 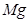 +
+ 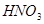 ����
����
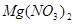 +
+ 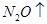 +
+
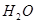
��16�֣�ÿ��2�֣�(1) �������ڵڢ�A�壬 Ca(OH)2��Һ ����
(2) 2OH- + Mg2+ �� Mg(OH)2��
(3) 
(4) MgCl2(����) Mg
+ Cl2�� (5)2K + MgO
Mg
+ Cl2�� (5)2K + MgO  K2O
+ Mg
K2O
+ Mg
(6) 1��2��1��2 4��10��4��1��5
��������
�����������1��þ��ԭ��������12��λ��Ԫ�����ڱ��ĵ������ڵڢ�A�塣��ˮ�е�þ����ת��������Ӧ�ü���ʯ�����������ƣ���þ����ת��Ϊ������þ������������þת��Ϊ�Ȼ�þ����Ҫ��������þ�ܽ��������С�
��2���������Ϸ�����֪������ٵ����ӷ���ʽ��2OH- + Mg2+��Mg(OH)2����
��3���Ȼ�þ�Ǻ������Ӽ������ӻ�������γɹ��̿ɱ�ʾΪ
 ��
��
��4����ͨ��������£�������ڵ��Ȼ�þ���������ͽ���þ����Ӧ�ķ���ʽ��MgCl2(����) Mg
+ Cl2����
Mg
+ Cl2����
��5���ڸ����£�����K������þ�����û���Ӧ�����ɽ���þ����Ӧ�Ļ�ѧ����ʽ��2K
+ MgO  K2O
+ Mg��
K2O
+ Mg��
��6�����ڷ�Ӧ��Mg�Ļ��ϼ۴�0�����ߵ���2�ۣ�ʧȥ2�����ӡ�UԪ�صĻ��ϼ۴ӣ�4�۽��͵�0�ۣ��õ�4�����ӣ����Ը��ݵ��ӵĵ�ʧ�غ��֪����ƽ��ķ���ʽ�� 1  + 2
+ 2
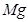 �� 1
�� 1 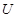 + 2
+ 2  ��
��
�ڸ��ݷ���ʽ��֪��Mg�Ļ��ϼ۴�0�����ߵ���2�ۣ�ʧȥ2�����ӡ������е�Ԫ�صĻ��ϼ۴ӣ�5�۽��͵���1�ۣ��õ�4�����ӣ�����ݵ��ӵĵ�ʧ�غ��֪����ƽ��ķ���ʽ��
4 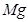 + 10
+ 10 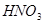 ��
4
��
4 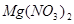 + 1
+ 1
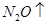 +
5
+
5 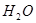 ��
��
���㣺���麣ˮ�н���þ����ȡ������þ�����ڱ��е�λ�á��Ȼ�þ���γɡ�������ԭ��Ӧ����ʽ����ƽ
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ����Ӧ�������������������������Ժ�ˮ���ۺ�Ӧ��Ϊ���壬�����ڵ���ѧ����ѧϰ��Ȥ������ѧ����ѧϰ��֪����