��Ŀ����
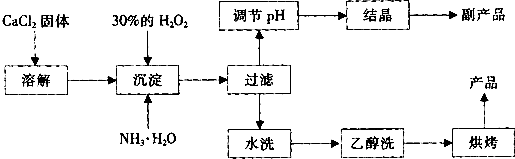
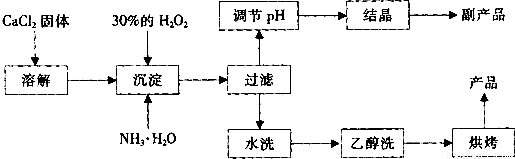
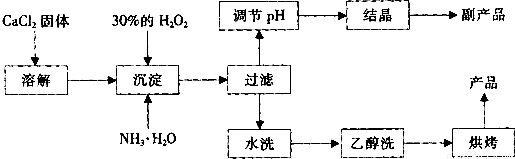
�����Ĺ�������(CaO2)������ˮ���Ҵ��������½�Ϊ�ȶ���CaO2��8H2O��0��ʱ�ȶ���������130��ʱ��Ϊ��ˮCaO2����ʵ���ҿ��ø�����ȡCaO2��8H2O���پ���ˮ�Ƶ�CaO2�����Ʊ���������

����������Ϣ���ش��������⣺
(1)������������ȡCaO2��8H2O�Ļ�ѧ����ʽ��________________________��
(2)����A�IJ���Ϊ______________________��
(3)���顰ˮϴ���Ѿ�ϴ���ķ�����_____________________��
(4)�ⶨ��Ʒ��CaO2�ĺ�����ʵ�鲽���ǣ���֪��I2 + 2S2O32- = 2I- + S4O62-����
��һ����ȷ��ȡag��Ʒ����ƿ�У�������������ˮ������bg KI���壬�ٵ�������2 mol/L��H2SO4
��Һ����ַ�Ӧ��
�ڶ�������������ƿ�м��뼸�ε�����Һ��
����������μ���Ũ��Ϊc mol/L��Na2S2O3��Һ����Ӧ��ȫ������Na2S2O3��ҺVmL��
�ٵ�������Ӧ��ȫʱ������Ϊ______________________��
�ڲ�Ʒ��CaO2����������Ϊ___________������ĸ��ʾ����
��ijͬѧ��ʵ���ò�Ʒ��CaO2����������ƫ�ߣ����ƫ�ߵ�ԭ���ǣ��ⶨ�������ɲ��������������Բ��ƣ������ӷ���ʽ��ʾ��______________________��
(1)������������ȡCaO2��8H2O�Ļ�ѧ����ʽ��________________________��
(2)����A�IJ���Ϊ______________________��
(3)���顰ˮϴ���Ѿ�ϴ���ķ�����_____________________��
(4)�ⶨ��Ʒ��CaO2�ĺ�����ʵ�鲽���ǣ���֪��I2 + 2S2O32- = 2I- + S4O62-����
��һ����ȷ��ȡag��Ʒ����ƿ�У�������������ˮ������bg KI���壬�ٵ�������2 mol/L��H2SO4
��Һ����ַ�Ӧ��
�ڶ�������������ƿ�м��뼸�ε�����Һ��
����������μ���Ũ��Ϊc mol/L��Na2S2O3��Һ����Ӧ��ȫ������Na2S2O3��ҺVmL��
�ٵ�������Ӧ��ȫʱ������Ϊ______________________��
�ڲ�Ʒ��CaO2����������Ϊ___________������ĸ��ʾ����
��ijͬѧ��ʵ���ò�Ʒ��CaO2����������ƫ�ߣ����ƫ�ߵ�ԭ���ǣ��ⶨ�������ɲ��������������Բ��ƣ������ӷ���ʽ��ʾ��______________________��
(1) CaCl2+H2O2+2NH3��H2O+6H2O=CaO2��8H2O��+2NH4Cl
(2)����Һ�м�������HCl������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ�����
(3)ȡ�������һ��ϴ��Һ�������м���ϡ�����ữ����������Һ������������ɫ��������˵���Ѿ�ϴ�� (4)����Һ��ɫ��ȥ����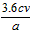 %����4H+ +4I- +O2 = 2I2 +2H2O
%����4H+ +4I- +O2 = 2I2 +2H2O
(2)����Һ�м�������HCl������Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ�����
(3)ȡ�������һ��ϴ��Һ�������м���ϡ�����ữ����������Һ������������ɫ��������˵���Ѿ�ϴ�� (4)����Һ��ɫ��ȥ����
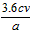 %����4H+ +4I- +O2 = 2I2 +2H2O
%����4H+ +4I- +O2 = 2I2 +2H2O 
��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ