��Ŀ����
��2010?��ƽ��ģ�⣩�����Ĺ������ƣ�CaO2���ǰ�ɫ�Ľᾧ��ĩ��������ˮ���������Ҵ������ѣ������½�Ϊ�ȶ�����һ������ˮ����ֳ���������������ʻ�ˮ��Ʒ�����䣮
��֪�����ڳ�ʪ������CaO2�ܹ�������Ӧ��CaO2+2H2O��Ca��OH��2+H2O2 2CaO2+2CO2��2CaCO3+O2
��CaO2��ϡ�ᷴӦ�����κ�H2O2��CaO2+2H+��Ca2++H2O2
��ʵ���ҿ��ø�����ȡCaO2?8H2O���پ���ˮ�Ƶ�CaO2��CaO2?8H2O��0��ʱ�ȶ���������ʱ��������ͷֽ⣬������130��ʱ��Ϊ��ˮCaO2��
���Ʊ��������£�

����������Ϣ���ش��������⣺
��1��������������ȡCaO2?8H2O�Ļ�ѧ����ʽ��
��2��Ϊ�˿��Ƴ����¶�Ϊ0�����ң���ʵ�����˲�ȡ�ķ�����
��3�����Ʒ��ĸ���ƷΪ
��4��Ϊ�˼��顰ˮϴ���Ƿ�ϸ�ȡ����ϴ��Һ���Թ��У��ٵμ�
��5���ⶨ��Ʒ��CaO2�ĺ�����ʵ�鲽���ǣ�
��һ����ȷ��ȡa g��Ʒ��������ƿ�У�������������ˮ������b g KI���壬�ٵ�������2mol/L��H2SO4��Һ����ַ�Ӧ��
�ڶ�������������ƿ�м��뼸�ε�����Һ��
����������μ���Ũ��Ϊc mol/L��Na2S2O3��Һ����Ӧ��ȫ������Na2S2O3��ҺV mL������֪��I2+2S2O32-��2I-+S4O62-����ɫ����
�ٵ�������˵����Ӧǡ����ȫ��������
��CaO2����������Ϊ
������ĸ��ʾ����
��֪�����ڳ�ʪ������CaO2�ܹ�������Ӧ��CaO2+2H2O��Ca��OH��2+H2O2 2CaO2+2CO2��2CaCO3+O2
��CaO2��ϡ�ᷴӦ�����κ�H2O2��CaO2+2H+��Ca2++H2O2
��ʵ���ҿ��ø�����ȡCaO2?8H2O���پ���ˮ�Ƶ�CaO2��CaO2?8H2O��0��ʱ�ȶ���������ʱ��������ͷֽ⣬������130��ʱ��Ϊ��ˮCaO2��
���Ʊ��������£�

����������Ϣ���ش��������⣺
��1��������������ȡCaO2?8H2O�Ļ�ѧ����ʽ��
CaCl2+H2O2+2NH3+8H2O=CaO2?8H2O��+2NH4Cl����CaCl2+H2O2+2NH3?H2O+6H2O=CaO2?8H2O��+2NH4Cl
CaCl2+H2O2+2NH3+8H2O=CaO2?8H2O��+2NH4Cl����CaCl2+H2O2+2NH3?H2O+6H2O=CaO2?8H2O��+2NH4Cl
����2��Ϊ�˿��Ƴ����¶�Ϊ0�����ң���ʵ�����˲�ȡ�ķ�����
��ˮԡ��ȴ����Ӧ���������ڱ�ˮ�У�
��ˮԡ��ȴ����Ӧ���������ڱ�ˮ�У�
����3�����Ʒ��ĸ���ƷΪ
NH4Cl
NH4Cl
���ѧʽ����Ϊ����߸���Ʒ�IJ��ʣ��ᾧǰҪ����Һ��pH���������ʷ�Χ���ɼ�����Լ���A
A
�� A������ B����ˮ��4��Ϊ�˼��顰ˮϴ���Ƿ�ϸ�ȡ����ϴ��Һ���Թ��У��ٵμ�
ϡ�����ữ����������Һ
ϡ�����ữ����������Һ
����5���ⶨ��Ʒ��CaO2�ĺ�����ʵ�鲽���ǣ�
��һ����ȷ��ȡa g��Ʒ��������ƿ�У�������������ˮ������b g KI���壬�ٵ�������2mol/L��H2SO4��Һ����ַ�Ӧ��
�ڶ�������������ƿ�м��뼸�ε�����Һ��
����������μ���Ũ��Ϊc mol/L��Na2S2O3��Һ����Ӧ��ȫ������Na2S2O3��ҺV mL������֪��I2+2S2O32-��2I-+S4O62-����ɫ����
�ٵ�������˵����Ӧǡ����ȫ��������
��Һ����ɫ��Ϊ��ɫ����30s���ָ�
��Һ����ɫ��Ϊ��ɫ����30s���ָ�
����CaO2����������Ϊ
| 36cV��10-3 |
| a |
| 36cV��10-3 |
| a |
��������1��ʵ���Ŀ��Ϊ�Ʊ�CaO2?8H2O���������еij���ӦΪCaO2?8H2O�����������غ��жϻ�Ӧ��NH4Cl���ɣ����������غ㶨�ɿ�д����Ӧ�Ļ�ѧ����ʽ��
��2��CaO2?8H2O��0��ʱ�ȶ���Ϊ�˿��Ƴ����¶�Ϊ0�����ң���ʵ�����˲�ȡ�ķ����DZ�ˮԡ��ȴ���Դﵽʵ��Ŀ�ģ�
��3����Ӧ���а�ˮ������Ϊ��ֻ��ո���Ʒ��Ӧ�����������գ�
��4���ݼ���Cl-���ӵķ���������ϡ�����ữ����������Һ���飻
��5�����ݷ�Ӧ�����ӷ���ʽ��CaO2+4H++2I-�TCa2++2H2O+I2��I2+2S2O32-��2I-+S4O62-���ɵù�ϵʽCaO2��2S2O32-�����Դ˽��м��㣮
��2��CaO2?8H2O��0��ʱ�ȶ���Ϊ�˿��Ƴ����¶�Ϊ0�����ң���ʵ�����˲�ȡ�ķ����DZ�ˮԡ��ȴ���Դﵽʵ��Ŀ�ģ�
��3����Ӧ���а�ˮ������Ϊ��ֻ��ո���Ʒ��Ӧ�����������գ�
��4���ݼ���Cl-���ӵķ���������ϡ�����ữ����������Һ���飻
��5�����ݷ�Ӧ�����ӷ���ʽ��CaO2+4H++2I-�TCa2++2H2O+I2��I2+2S2O32-��2I-+S4O62-���ɵù�ϵʽCaO2��2S2O32-�����Դ˽��м��㣮
����⣺��1����ʵ���Ŀ��Ϊ�Ʊ�CaO2?8H2O���������еij���ӦΪCaO2?8H2O�����������غ��жϻ�Ӧ��NH4Cl���ɣ��ʿ�д����Ӧ�Ļ�ѧ����ʽΪ��CaCl2+H2O2+2NH3+8H2O=CaO2?8H2O��+2NH4Cl����CaCl2+H2O2+2NH3?H2O+6H2O=CaO2?8H2O��+2NH4Cl���ʴ�Ϊ��CaCl2+H2O2+2NH3+8H2O=CaO2?8H2O��+2NH4Cl����CaCl2+H2O2+2NH3?H2O+6H2O=CaO2?8H2O��+2NH4Cl��
��2��CaO2?8H2O��0��ʱ�ȶ���Ϊ�˿��Ƴ����¶�Ϊ0�����ң���ʵ�����˲�ȡ�ķ����DZ�ˮԡ��ȴ���Դﵽʵ��Ŀ�ģ��ʴ�Ϊ����ˮԡ��ȴ����Ӧ���������ڱ�ˮ�У�
��3�����ݷ�Ӧ��Ӧ����ʽ���жϸ÷�Ӧ����CaO2?8H2O��NH4Cl����Ӧ���а�ˮ������Ϊ��ֻ��ո���Ʒ��Ӧ�����������գ��ʴ�Ϊ��NH4Cl��A��
��4����Һ�к��д�����Cl-���ӣ�Ϊ������ϴ�Ӹɾ���Ӧ���ϴ�ӣ����ݼ���Cl-���ӵķ���������ϡ�����ữ����������Һ���飬��Ӧ�����ӷ���ʽΪ��Cl-+Ag+�TAgCl�����ʴ�Ϊ��ϡ�����ữ����������Һ��
��5����CaO2����ǿ�����ԣ���Һ�м���KI����͵�����Һ�����ɵĵⵥ�������۱���ɫ����Ӧ�����ӷ���ʽΪ��CaO2+4H++2I-�TCa2++2H2O+I2���ʴ�Ϊ����Һ����ɫ��Ϊ��ɫ����30s���ָ���
�ڸ��ݷ�Ӧ�����ӷ���ʽ��CaO2+4H++2I-�TCa2++2H2O+I2��I2+2S2O32-��2I-+S4O62-���ɵù�ϵʽ���Դ˽��м��㣺
CaO2��2S2O32-
72g 2mol
m cV��10-3mol
m=
=36cV��10-3g
��CaO2����������Ϊ
�ʴ�Ϊ��
��
��2��CaO2?8H2O��0��ʱ�ȶ���Ϊ�˿��Ƴ����¶�Ϊ0�����ң���ʵ�����˲�ȡ�ķ����DZ�ˮԡ��ȴ���Դﵽʵ��Ŀ�ģ��ʴ�Ϊ����ˮԡ��ȴ����Ӧ���������ڱ�ˮ�У�
��3�����ݷ�Ӧ��Ӧ����ʽ���жϸ÷�Ӧ����CaO2?8H2O��NH4Cl����Ӧ���а�ˮ������Ϊ��ֻ��ո���Ʒ��Ӧ�����������գ��ʴ�Ϊ��NH4Cl��A��
��4����Һ�к��д�����Cl-���ӣ�Ϊ������ϴ�Ӹɾ���Ӧ���ϴ�ӣ����ݼ���Cl-���ӵķ���������ϡ�����ữ����������Һ���飬��Ӧ�����ӷ���ʽΪ��Cl-+Ag+�TAgCl�����ʴ�Ϊ��ϡ�����ữ����������Һ��
��5����CaO2����ǿ�����ԣ���Һ�м���KI����͵�����Һ�����ɵĵⵥ�������۱���ɫ����Ӧ�����ӷ���ʽΪ��CaO2+4H++2I-�TCa2++2H2O+I2���ʴ�Ϊ����Һ����ɫ��Ϊ��ɫ����30s���ָ���
�ڸ��ݷ�Ӧ�����ӷ���ʽ��CaO2+4H++2I-�TCa2++2H2O+I2��I2+2S2O32-��2I-+S4O62-���ɵù�ϵʽ���Դ˽��м��㣺
CaO2��2S2O32-
72g 2mol
m cV��10-3mol
m=
| 72g��cV��10-3mol |
| 2mol |
��CaO2����������Ϊ
| 36cV��10-3 |
| a |
�ʴ�Ϊ��
| 36cV��10-3 |
| a |
���������⿼����ʽΪ�����Ʊ�����ͼ��Ŀ���漰���ʵĻ�ѧ����ʽ����д��ʵ�鷽����ʵ����������ʵļ���ͼ�������⣬����ʱע��������عؼ���Ϣ������ʵ��������������⣬�����Ϊ�ۺϣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��2010?��ƽ��ģ�⣩�±��Գ��������ȷ�Լ������������ϵ���ж϶���ȷ���ǣ�������
|
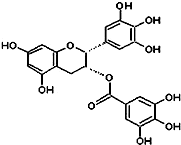 ��2010?��ƽ��ģ�⣩�̲��к���EGCG����ʾûʳ�Ӷ�����ûʳ�����������ʾ��п������ã���֪EGCG��һ��������A��ͼ��ʾ���й�������A˵������ȷ���ǣ�������
��2010?��ƽ��ģ�⣩�̲��к���EGCG����ʾûʳ�Ӷ�����ûʳ�����������ʾ��п������ã���֪EGCG��һ��������A��ͼ��ʾ���й�������A˵������ȷ���ǣ�������
 CH3CH2OH��g��+3H2O��g�� 25��ʱ��K=2.95��1011
CH3CH2OH��g��+3H2O��g�� 25��ʱ��K=2.95��1011 CH3CH2OH��g��+H2O��g�� 25��ʱ��K=1.71��1022
CH3CH2OH��g��+H2O��g�� 25��ʱ��K=1.71��1022