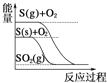
جâؤ؟ؤعبف
،¾جâؤ؟،؟¶رُ»¯آب(ClO2))تاز»ضضشعث®´¦ہيµب·½أوسذ¹م·؛س¦سأµؤ¸كذ§°²ب«دû¶¾¼ء£¬¶ّازسëCl2دà±ب²»»ل²ْةْ¶شبثجهسذا±شعخ£؛¦µؤسذ»ْآب´ْخï،£ؤ³؟خجâ×éزش¶èذشµç¼«µç½âرخثل؛حNH4Clµؤ»ى؛دبـز؛»ٌµأNCl3بـز؛£¬شظزشNCl3بـز؛؛حNaClO2·´س¦ضئµأClO2،£»ط´ًدآءذختجâ£؛
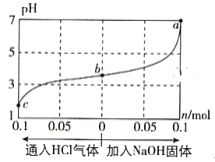
£¨1£©ClO2±»I-»¹شخھClO2-،¢Cl-µؤ×ھ»¯آتسëبـز؛pHµؤ¹طدµبçح¼ثùت¾£؛
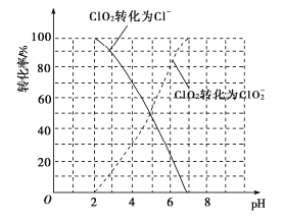
¢ظpH،ـ2ت±£¬ClO2سëI-·´س¦ةْ³ةI2µؤہë×س·½³جت½خھ_________________،£
¢عشعسأClO2½ّذذث®´¦ہيت±£¬³ءثة±¾ْدû¶¾ح⣬»¹ؤـ³ب¥ث®ضذµؤFe2+؛حMn2+£¬ClO2رُ»¯Mn2+ةْ³ةMnO2µؤ·´س¦ضذ£¬رُ»¯¼ء؛ح»¹ش¼ءµؤخïضتµؤء؟ض®±بخھ________،£
£¨2£©NCl3µؤث®½â²ْخïسذNHCl2،¢NH2Clµب،£
¢ظNCl3µؤµç×ست½خھ________£¬ئنضذآبشھثطµؤ»¯؛د¼غتا________£»NH2Clبش؟ة»؛آ·¢ةْث®½â£¬ئن»¯ر§·½³جت½خھ_________________،£
¢عNCl3شعNaOHبـز؛ضذث®½âةْ³ةN2£¬NaClO؛حNaCl£¬ئن»¯ر§·½³جت½خھ______________،£
¢غNCl3سëNaClO2°´خïضتµؤء؟ض®±ب1،أ6»ى؛د£¬شعبـز؛ضذا،؛أ·´س¦ةْ³ةClO2؛ح°±£¬¸أ·´س¦µؤہë×س·½³جت½خھ___________________،£
£¨3£©سذدآءذء½ضض·½·¨ضئ±¸ClO2£؛
·½·¨ز»£؛2NaClO3+4HCl=2ClO2،ü+Cl2،ü+2NaCl+2H2O
·½·¨¶£؛2NaClO3+H2O2+H2SO4=2ClO2،ü+Na2SO4+O2،ü+2H2O
سأ·½·¨¶ضئ±¸µؤClO2¸üتت؛دسأسعزûسأث®دû¶¾£¬ئنض÷زھشزٍتا_______________،£
£¨4£©µç½â»ٌµأNCl3بـز؛µؤ»¯ر§·½³جت½خھ__________________،£
،¾´ً°¸،؟2ClO2+10I-+8H+=2Cl-+5I2+4H2O 2£؛1 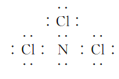 +1 NH2Cl+2H2O
+1 NH2Cl+2H2O ![]() NH3،¤H2O+HClO 2NCl3+6NaOH=N2،ü+3NaCl+3NaClO+3H3O NCl3+6ClO-+3H2O=6ClO2،ü+NH3،ü+3Cl-+3OH- ضئ±¸µؤClO2ضذ²»؛¬Cl2£¬²»»ل²ْةْ¶شبثجهسذا±شعخ£؛¦µؤسذ»ْآب´ْخï NH4Cl+2HCl
NH3،¤H2O+HClO 2NCl3+6NaOH=N2،ü+3NaCl+3NaClO+3H3O NCl3+6ClO-+3H2O=6ClO2،ü+NH3،ü+3Cl-+3OH- ضئ±¸µؤClO2ضذ²»؛¬Cl2£¬²»»ل²ْةْ¶شبثجهسذا±شعخ£؛¦µؤسذ»ْآب´ْخï NH4Cl+2HCl ![]() 3H2،ü+NCl3
3H2،ü+NCl3
،¾½âخِ،؟
£¨1£©¢ظسةح¼؟ةضھ£¬pH،ـ2ت±£¬ClO2سëI£·´س¦ةْ³ةI2،¢Cl-؛حH2O£»
¢عسةح¼؟ةضھ£¬ضذذشبـز؛ضذClO2سëMn2+·´س¦ةْ³ةMnO2؛حClO2-£»NH2Clشعبـز؛ضذ»؛آ·¢ةْث®½âةْ³ةز»ث®؛د°±؛ح´خآبثل£»
£¨2£©¢ظسةNCl3µؤث®½â²ْخïسذNHCl2،¢NH2Cl؟ةضھ£¬·ض×سضذµھشھثط³ت-3¼غ£¬آبشھثط³ت+1¼غ£»
¢عNCl3شعNaOHبـز؛ضذث®½âةْ³ةN2£¬NaClO؛حNaCl£¬·´س¦ضذµھشھثط±»رُ»¯£¬آبشھثط²؟·ض±»»¹ش£»
¢غسةNCl3سëNaClO2°´خïضتµؤء؟ض®±ب1،أ6»ى؛د£¬شعبـز؛ضذا،؛أ·´س¦ةْ³ةClO2؛ح°±؟ةضھ£¬·´س¦ضذ+3¼غآبشھثطت§µç×س±»رُ»¯ةْ³ةClO2£¬+1¼غآبشھثطµأµç×س±»»¹شةْ³ةCl-£»
£¨4£©سةجâ¸ّذإد¢؟ةضھCl2شعث®´¦ہي¹³جضذ»ل²ْةْ¶شبثجهسذا±شعخ£؛¦µؤسذ»ْآب´ْخسأ·½·¨¶ضئ±¸µؤClO2ضذ²»؛¬Cl2£»
£¨5£©زش¶èذشµç¼«µç½âرخثل؛حNH4Clµؤ»ى؛دبـز؛»ٌµأNCl3بـز؛ت±£¬آبہë×سشعرô¼«ت§µç×س·¢ةْرُ»¯·´س¦ةْ³ةNCl3£¬اâہë×سشعزُ¼«µأµç×س·¢ةْ»¹ش·´س¦ةْ³ةاâئّ،£
£¨1£©¢ظسةح¼؟ةضھ£¬pH،ـ2ت±£¬ClO2سëI-·´س¦ةْ³ةI2،¢Cl-؛حH2O£¬·´س¦µؤہë×س·½³جت½خھ2ClO2+10I-+8H+=2Cl-+5I2+4H2O£¬¹ت´ً°¸خھ2ClO2+10I-+8H+=2Cl-+5I2+4H2O£»
¢عسةح¼؟ةضھ£¬ضذذشبـز؛ضذClO2سëMn2+·´س¦ةْ³ةMnO2؛حClO2-£¬سةµأت§µç×ستؤ؟تط؛م؟ةضھ£¬ClO2رُ»¯Mn2+ةْ³ةMnO2µؤ·´س¦ضذسذبçدآ¹طدµn(ClO2)=2n(Mn2+)£¬شٍرُ»¯¼ء؛ح»¹ش¼ءµؤخïضتµؤء؟ض®±بخھ2:1£¬¹ت´ً°¸خھ2:1£»
£¨2£©¢ظNCl3خھ¹²¼غ»¯؛دخسةNCl3µؤث®½â²ْخïسذNHCl2،¢NH2Cl؟ةضھ£¬·ض×سضذµھشھثط³ت،ھ3¼غ£¬آبشھثط³ت+1¼غ£¬µç×ست½خھ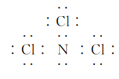 £»NH2Clشعبـز؛ضذ»؛آ·¢ةْث®½âةْ³ةز»ث®؛د°±؛ح´خآبثل£¬ث®½â·´س¦µؤ»¯ر§·½³جت½خھNH2Cl+2H2O
£»NH2Clشعبـز؛ضذ»؛آ·¢ةْث®½âةْ³ةز»ث®؛د°±؛ح´خآبثل£¬ث®½â·´س¦µؤ»¯ر§·½³جت½خھNH2Cl+2H2O ![]() NH3،¤H2O+HClO£¬¹ت´ً°¸خھ
NH3،¤H2O+HClO£¬¹ت´ً°¸خھ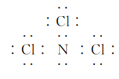 £»+1£»NH2Cl+2H2O
£»+1£»NH2Cl+2H2O ![]() NH3،¤H2O+HClO£»
NH3،¤H2O+HClO£»
¢عNCl3شعNaOHبـز؛ضذث®½âةْ³ةN2£¬NaClO؛حNaCl£¬·´س¦ضذµھشھثط±»رُ»¯£¬آبشھثط²؟·ض±»»¹ش£¬·´س¦µؤ»¯ر§·½³جت½خھ2NCl3+6NaOH=N2،ü+3NaCl+3NaClO+3H3O£¬¹ت´ً°¸خھ2NCl3+6NaOH=N2،ü+3NaCl+3NaClO+3H3O£»
¢غسةNCl3سëNaClO2°´خïضتµؤء؟ض®±ب1،أ6»ى؛د£¬شعبـز؛ضذا،؛أ·´س¦ةْ³ةClO2؛ح°±؟ةضھ£¬·´س¦ضذ+3¼غآبشھثطت§µç×س±»رُ»¯ةْ³ةClO2£¬+1¼غآبشھثطµأµç×س±»»¹شةْ³ةCl-£¬·´س¦µؤہë×س·½³جت½خھNCl3+6ClO2-+3H2O=6ClO2،ü+NH3،ü+3Cl-+3OH-£¬¹ت´ً°¸خھNCl3+6ClO-+3H2O=6ClO2،ü+NH3،ü+3Cl-+3OH-£»
£¨4£©سةجâ¸ّذإد¢؟ةضھCl2شعث®´¦ہي¹³جضذ»ل²ْةْ¶شبثجهسذا±شعخ£؛¦µؤسذ»ْآب´ْخسأ·½·¨¶ضئ±¸µؤClO2ضذ²»؛¬Cl2£¬²»»ل²ْةْ¶شبثجهسذا±شعخ£؛¦µؤسذ»ْآب´ْخ¹تر،شٌ·½·¨¶£¬¹ت´ً°¸خھضئ±¸µؤClO2ضذ²»؛¬Cl2£¬²»»ل²ْةْ¶شبثجهسذا±شعخ£؛¦µؤسذ»ْآب´ْخ
£¨5£©زش¶èذشµç¼«µç½âرخثل؛حNH4Clµؤ»ى؛دبـز؛»ٌµأNCl3بـز؛ت±£¬آبہë×سشعرô¼«ت§µç×س·¢ةْرُ»¯·´س¦ةْ³ةNCl3£¬اâہë×سشعزُ¼«µأµç×س·¢ةْ»¹ش·´س¦ةْ³ةاâئّ£¬µç½â·´س¦µؤ»¯ر§·½³جت½خھNH4Cl+2HCl ![]() 3H2،ü+NCl3£¬¹ت´ً°¸خھNH4Cl+2HCl
3H2،ü+NCl3£¬¹ت´ً°¸خھNH4Cl+2HCl ![]() 3H2،ü+NCl3،£
3H2،ü+NCl3،£

،¾جâؤ؟،؟اë°´زھاَ»ط´ًدآءذختجâ£؛
£¨1£©´؟ث®شع100،وت±pH£½6£¬¸أخآ¶بدآ1mol،¤L-1µؤNaOHبـز؛ضذ£¬سةث®µçہë³ِµؤc(OH£)=__mol،¤L-1،£
£¨2£©25،وت±£¬دٍث®µؤµçہëئ½؛âجهدµضذ¼سبëةظء؟ج¼ثلؤئ¹ججه£¬µأµ½pHخھ11µؤبـز؛£¬ئنث®½âµؤہë×س·½³جت½خھ__£¬سةث®µçہë³ِµؤc(OH£)£½___mol،¤L£1،£
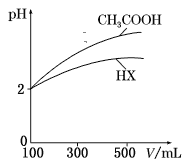
£¨3£©جه»¾ùخھ100 mL،¢pH¾ùخھ2µؤCH3COOHسëز»شھثلHX£¬¼سث®د،تح¹³جضذpHسëبـز؛جه»µؤ¹طدµبçح¼ثùت¾£¬شٍHXµؤµçہë³£ت___(جî،°´َسع،±،°ذ،سع،±»ٍ،°µبسع،±)CH3COOHµؤµçہë³£ت،£ہيسةتا___،£
£¨4£©µçہë³£تتا؛âء؟بُµç½âضتµçہë³ج¶با؟بُµؤخïہيء؟،£زرضھ£؛
»¯ر§ت½ | µçہë³£ت(25،و) |
HCN | K£½4.9،ء10£10 |
CH3COOH | K£½1.8،ء10£5 |
H2CO3 | K1£½4.3،ء10£7،¢K2£½5.6،ء10£11 |
¢ظ25،وت±£¬سذµبpHµؤa.NaCNبـز؛،¢b.Na2CO3بـز؛؛حc.CH3COONaبـز؛£¬ببـز؛µؤإ¨¶بسة´َµ½ذ،µؤث³ذٍخھ___،£(سأa،¢b،¢c±يت¾)
¢عدٍNaCNبـز؛ضذح¨بëةظء؟µؤCO2£¬·¢ةْ·´س¦µؤ»¯ر§·½³جت½خھ___،£
¢غ25،وت±£¬µبإ¨¶بµؤHCN؛حNaCN»ى؛دبـز؛دش___ذش،££¨ثل،¢¼î،¢ضذ£©
£¨5£©تزخآت±£¬دٍ100mL0.1mol/LNH4HSO4بـز؛ضذµخ¼س0.1mol/LNaOHبـز؛£¬µأµ½بـز؛pHسëNaOHبـز؛جه»µؤ¹طدµاْدكبçح¼ثùت¾£؛
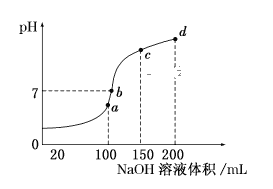
تش·ضخِح¼ضذa،¢b،¢c،¢dثؤ¸ِµم£¬ث®µؤµçہë³ج¶ب×î´َµؤتا__£»شعbµم£¬بـز؛ضذ¸÷ہë×سإ¨¶بسة´َµ½ذ،µؤإإءذث³ذٍتا__،£