��Ŀ����
SO2��һ����Ҫ�Ļ���ԭ�ϣ�Ҳ������������Ⱦ����Ҫ��Դ��
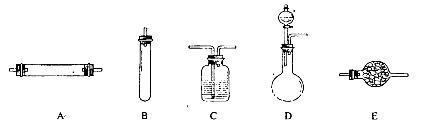
(1)ij��ȤС�������ͼ��ʾװ����ȡSO2
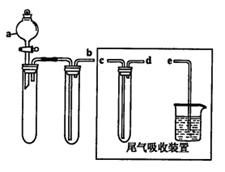
������ʵ�鷽��������ͼ��ʾװ����ȡ����SO2���Լ���_______(����ţ���
��a������������_______��
��β������װ�õ�����˳����b��( )�� ( )��e��
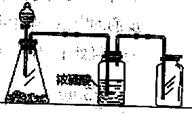
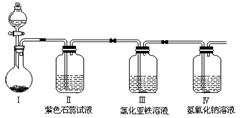
(2)Ϊ�˻�������SO2�������о���Ա���������õ�Ʒλ���̿�(��Ҫ�ɷ���MnO2)���ո��±��պ������������SO2�������Ʊ������̾���(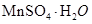 �������̣�������ʾ��ͼ���£�
�������̣�������ʾ��ͼ���£�
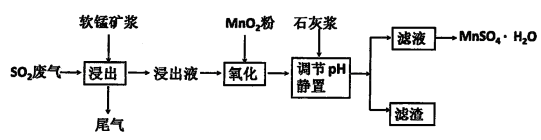
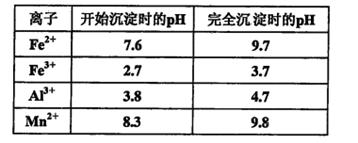
��֪������Һ��pH<2�����еĽ���������Ҫ��Mn2+��������������Fe2����Al3���������������ӡ��йؽ��������γ������������ʱ��Һ��pH���±���
��ش�
�ٺ�Al3�����γ�������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��________________________��
�ڽ�����������Ҫ��Ӧ�Ļ�ѧ����ʽ��___________________________________________��
�����������м���MnO2�۵�Ŀ����______________________________________________;
��Ӧ�����ӷ���ʽ��_________________________________________________________��
����ʯ�ҽ�����pH��pHӦ���ڵķ�Χ��___________________________________��
����������Ҫ�ɷ���____________________________________��
(1)ij��ȤС�������ͼ��ʾװ����ȡSO2

������ʵ�鷽��������ͼ��ʾװ����ȡ����SO2���Լ���_______(����ţ���
| A��Na2SO3��Һ��ϡ���� |
| B��Na2SO3������Ũ���� |
| C������������� |
| D��ͭ��Ũ���� |
��β������װ�õ�����˳����b��( )�� ( )��e��
(2)Ϊ�˻�������SO2�������о���Ա���������õ�Ʒλ���̿�(��Ҫ�ɷ���MnO2)���ո��±��պ������������SO2�������Ʊ������̾���(
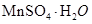 �������̣�������ʾ��ͼ���£�
�������̣�������ʾ��ͼ���£�
��֪������Һ��pH<2�����еĽ���������Ҫ��Mn2+��������������Fe2����Al3���������������ӡ��йؽ��������γ������������ʱ��Һ��pH���±���

��ش�
�ٺ�Al3�����γ�������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��________________________��
�ڽ�����������Ҫ��Ӧ�Ļ�ѧ����ʽ��___________________________________________��
�����������м���MnO2�۵�Ŀ����______________________________________________;
��Ӧ�����ӷ���ʽ��_________________________________________________________��
����ʯ�ҽ�����pH��pHӦ���ڵķ�Χ��___________________________________��
����������Ҫ�ɷ���____________________________________��
��1����B (2��)
�ڷ�Һ©�� (2��)
��d c (2��)
��2����Al3++3H2O Al(OH)3+3H + (2��)
Al(OH)3+3H + (2��)
��SO2��MnO2��MnSO4 (2��)
�۽�Fe2������ΪFe3�� (2��)
2Fe2����MnO2��4H����2Fe3����Mn2����2H2O (2��)
��4.7��pH��8.3 (2��)
��������������������������� ��2�֣�
�ڷ�Һ©�� (2��)
��d c (2��)
��2����Al3++3H2O
 Al(OH)3+3H + (2��)
Al(OH)3+3H + (2��)��SO2��MnO2��MnSO4 (2��)
�۽�Fe2������ΪFe3�� (2��)
2Fe2����MnO2��4H����2Fe3����Mn2����2H2O (2��)
��4.7��pH��8.3 (2��)
��������������������������� ��2�֣�
��1���ٸ�װ�����ڹ�Һ��ҺҺ��ϲ�������װ��
A��Na2SO3��Һ��HNO3��Ӧʱ������������������Ʊ�������ԭΪһ����������A�����ϣ�
B��Na2SO3������Ũ������ȷ�Ӧ������Ũ�����ѻӷ��Կ��Է�Ӧ���ɶ����������壬��B���ϣ�
C����ȼ�ղ���Ҫ��Һ©������C�����ϣ�
D��ͭ��Ũ���ᷴӦ��Ҫ���ȣ���D�����ϣ�
��ѡB��
��a�����������ǣ���Һ©�����𰸣���Һ©����
����ͼ��β������װ�õ�����˳����b��(d)�� (c)��e���𰸣�d c ��

��2���ٺ�Al3�����γ�������ˮ����������Al3�� ˮ�������Al(OH)3���壬���������ԣ�����ˮ�е����������ʣ����ӷ���ʽΪ��Al3++3H2O Al(OH)3+3H + ���𰸣�Al3++3H2O
Al(OH)3+3H + ���𰸣�Al3++3H2O  Al(OH)3+3H + �� �ڵ�Ʒλ���̿���Ҫ�ɷ���MnO2��ͨ��SO2����Һ��pH��2�����еĽ���������Ҫ��Mn2������MnO2��SO2����������ԭ��Ӧ�Ļ�ѧ����ʽΪSO2+MnO2=MnSO4��
Al(OH)3+3H + �� �ڵ�Ʒλ���̿���Ҫ�ɷ���MnO2��ͨ��SO2����Һ��pH��2�����еĽ���������Ҫ��Mn2������MnO2��SO2����������ԭ��Ӧ�Ļ�ѧ����ʽΪSO2+MnO2=MnSO4��
�ʴ�Ϊ��SO2��MnO2��MnSO4 ��������������ֻ��Fe2�����л�ԭ�ԣ����Ա�MnO2������������������Fe3������Ӧ�����ӷ���ʽΪ2Fe2��+MnO2+4H��=2Fe3��+Mn2��+2H2O��
�ʴ�Ϊ����Fe2������ΪFe3�� 2Fe2����MnO2��4H����2Fe3����Mn2����2H2O ������Һ�м���ʯ�ҽ�������pH���������dz�ȥ�����к���Fe3����Al3�������ӣ���ͼ���Կ���������4.7���Խ�Fe3����Al3����ȥ��С��8.3�Ƿ�ֹMn2��Ҳ����������ֻҪ����pHֵ��4.7��8.3�伴�ɣ�
�ʴ�Ϊ��4.7��pH��8.3 ����Fe3����Al3��������ͨ����pHֵ��ת��Ϊ������������������������ͬʱ�����ܵ�����ƣ�����������Ҫ��������������������������ƣ�
�ʴ�Ϊ��������������������������� 2����
A��Na2SO3��Һ��HNO3��Ӧʱ������������������Ʊ�������ԭΪһ����������A�����ϣ�
B��Na2SO3������Ũ������ȷ�Ӧ������Ũ�����ѻӷ��Կ��Է�Ӧ���ɶ����������壬��B���ϣ�
C����ȼ�ղ���Ҫ��Һ©������C�����ϣ�
D��ͭ��Ũ���ᷴӦ��Ҫ���ȣ���D�����ϣ�
��ѡB��
��a�����������ǣ���Һ©�����𰸣���Һ©����
����ͼ��β������װ�õ�����˳����b��(d)�� (c)��e���𰸣�d c ��

��2���ٺ�Al3�����γ�������ˮ����������Al3�� ˮ�������Al(OH)3���壬���������ԣ�����ˮ�е����������ʣ����ӷ���ʽΪ��Al3++3H2O
 Al(OH)3+3H + ���𰸣�Al3++3H2O
Al(OH)3+3H + ���𰸣�Al3++3H2O  Al(OH)3+3H + �� �ڵ�Ʒλ���̿���Ҫ�ɷ���MnO2��ͨ��SO2����Һ��pH��2�����еĽ���������Ҫ��Mn2������MnO2��SO2����������ԭ��Ӧ�Ļ�ѧ����ʽΪSO2+MnO2=MnSO4��
Al(OH)3+3H + �� �ڵ�Ʒλ���̿���Ҫ�ɷ���MnO2��ͨ��SO2����Һ��pH��2�����еĽ���������Ҫ��Mn2������MnO2��SO2����������ԭ��Ӧ�Ļ�ѧ����ʽΪSO2+MnO2=MnSO4���ʴ�Ϊ��SO2��MnO2��MnSO4 ��������������ֻ��Fe2�����л�ԭ�ԣ����Ա�MnO2������������������Fe3������Ӧ�����ӷ���ʽΪ2Fe2��+MnO2+4H��=2Fe3��+Mn2��+2H2O��
�ʴ�Ϊ����Fe2������ΪFe3�� 2Fe2����MnO2��4H����2Fe3����Mn2����2H2O ������Һ�м���ʯ�ҽ�������pH���������dz�ȥ�����к���Fe3����Al3�������ӣ���ͼ���Կ���������4.7���Խ�Fe3����Al3����ȥ��С��8.3�Ƿ�ֹMn2��Ҳ����������ֻҪ����pHֵ��4.7��8.3�伴�ɣ�
�ʴ�Ϊ��4.7��pH��8.3 ����Fe3����Al3��������ͨ����pHֵ��ת��Ϊ������������������������ͬʱ�����ܵ�����ƣ�����������Ҫ��������������������������ƣ�
�ʴ�Ϊ��������������������������� 2����

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ