��Ŀ����
��11�֣�
��1�����±���һЩ������Ԫ�ص���̬ԭ��ʧȥ���ⲻͬ�������������(kJ��mol-1)��

ͨ�����е����ݷ���Ϊʲô�ԭ��ʧȥ����ڶ�������ʱ���������ҪԶԶ����ʧȥ��һ��������������� ��
X�����ڱ���λ�ã��� �ڣ� �壬Y���������Ϊ ��
��2��1932��������ѧ�ұ��֣�L��Pauling����������˵縺�Եĸ���縺�ԣ���X��ʾ��Ҳ��Ԫ�ص�һ����Ҫ���ʣ��±���������ԭ������С��20��16��Ԫ�صĵ縺����ֵ��
����ϸ�������ش������й����⣺
�١��ϱ��е縺����С��Ԫ���� (��Ԫ�ط���)�����Ƹ�Ԫ�صĵ縺�Ե�ȡֵ��Χ��__________��X��__________��
�ڡ�������ɸ������ǣ����γɻ�ѧ������ԭ����ӦԪ�صĵ縺�Բ�ֵ����1.7ʱ�����γɵ�һ��Ϊ���Ӽ�����С��1.7ʱ��һ��Ϊ���ۼ������ƶ�AlBr3���γɵĻ�ѧ��������Ϊ
______________���������� ��
��1�����±���һЩ������Ԫ�ص���̬ԭ��ʧȥ���ⲻͬ�������������(kJ��mol-1)��

ͨ�����е����ݷ���Ϊʲô�ԭ��ʧȥ����ڶ�������ʱ���������ҪԶԶ����ʧȥ��һ��������������� ��
X�����ڱ���λ�ã��� �ڣ� �壬Y���������Ϊ ��
��2��1932��������ѧ�ұ��֣�L��Pauling����������˵縺�Եĸ���縺�ԣ���X��ʾ��Ҳ��Ԫ�ص�һ����Ҫ���ʣ��±���������ԭ������С��20��16��Ԫ�صĵ縺����ֵ��
| Ԫ�� | H | Li | Be | B | C | N | O | F |
| �縺�� | 2.1 | 1.0 | 1.5] | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
| Ԫ�� | Na | Mg | Al | Si | P | S | Cl | K |
| �縺�� | 0.9 | 1.2 | 1.5 | 1.7 | 2.1 | 2.3 | 3.0 | 0.8 |
�١��ϱ��е縺����С��Ԫ���� (��Ԫ�ط���)�����Ƹ�Ԫ�صĵ縺�Ե�ȡֵ��Χ��__________��X��__________��
�ڡ�������ɸ������ǣ����γɻ�ѧ������ԭ����ӦԪ�صĵ縺�Բ�ֵ����1.7ʱ�����γɵ�һ��Ϊ���Ӽ�����С��1.7ʱ��һ��Ϊ���ۼ������ƶ�AlBr3���γɵĻ�ѧ��������Ϊ
______________���������� ��
��1����Liԭ��ʧȥһ�����Ӻ�Li+�Ѿ��γ����ȶ��ṹ����ʱ��ʧȥ���Ӻ����ѣ�2�֣��� 3��1�֣���IA��1�֣���+3��1�֣�
��2������ K ��0.8��X��1.2 ����1�֣���3�֣���
�ڡ����ۼ���1�֣�����ΪAlCl3��Cl��Al�ĵ縺�Բ�ֵΪ1.5����Br�ĵ縺��С��Cl������AlBr3����Ԫ�صĵ縺�Բ�ֵС��1.5��2�֣�
��2������ K ��0.8��X��1.2 ����1�֣���3�֣���
�ڡ����ۼ���1�֣�����ΪAlCl3��Cl��Al�ĵ縺�Բ�ֵΪ1.5����Br�ĵ縺��С��Cl������AlBr3����Ԫ�صĵ縺�Բ�ֵС��1.5��2�֣�
��1������Liԭ��ʧȥһ�����Ӻ�Li+�Ѿ��γ����ȶ��ṹ����ʱ��ʧȥ���Ӿ���Ҫ���ո���������������ԭ��ʧȥ����ڶ�������ʱ���������ҪԶԶ����ʧȥ��һ�����������������X�ĵڶ�������ԶԶ���ڴ��ڵ�һ�����ܣ�����X���ƣ�λ�ڵ������ڵ�IA��Y�ĵ��ĵ�����ԶԶ���ڵ��������ܣ�����Y�ǵڢ�AԪ�أ�����ǣ�3�ۡ�
��2���ٽ�����Խǿ���縺��ԽС�����Լصĵ縺����С��þ������ͬһ�������ڸƵĵ縺�Դ���þ�ġ��ƺͼ�����ͬһ���ڣ����ڸƵĵ縺��С�ڼصģ������䷶Χ��0.8��X��1.2��
�����ĵ縺����1.5��������λ����Ԫ�ص���һ���ڣ�������ĵ縺��С����Ԫ�صģ�����3.0���������С��3.0����˺����ĵ縺�Բ�ֵС��1.5�������γɵĻ�ѧ���Ǽ��Լ���
��2���ٽ�����Խǿ���縺��ԽС�����Լصĵ縺����С��þ������ͬһ�������ڸƵĵ縺�Դ���þ�ġ��ƺͼ�����ͬһ���ڣ����ڸƵĵ縺��С�ڼصģ������䷶Χ��0.8��X��1.2��
�����ĵ縺����1.5��������λ����Ԫ�ص���һ���ڣ�������ĵ縺��С����Ԫ�صģ�����3.0���������С��3.0����˺����ĵ縺�Բ�ֵС��1.5�������γɵĻ�ѧ���Ǽ��Լ���

��ϰ��ϵ�д�
 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
�����Ŀ
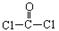 ÿ��COCl2�����ں��� ���ļ��� ���м���������ԭ�Ӳ�ȡ �ӻ������ʽ��
ÿ��COCl2�����ں��� ���ļ��� ���м���������ԭ�Ӳ�ȡ �ӻ������ʽ��