��Ŀ����
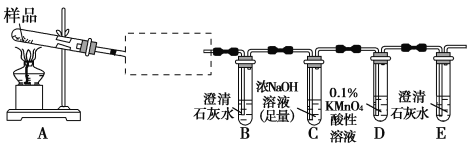
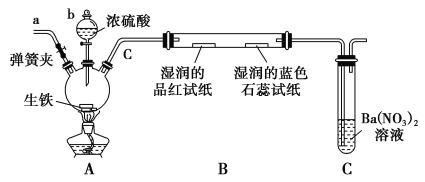
����Ŀ��������(AlN)��һ���������ǽ������ϣ�ijAlN��Ʒ������Al2O3���ʣ�Ϊ�ⶨAlN�ĺ������������ʵ�鷽����
(1)ʵ��ԭ����
AlN����Ũ��ǿ�����ɰ�������д��AlN��NaOH��Һ��Ӧ�Ļ�ѧ����_________��
(2)ʵ��װ�á�
��ͼCװ�������θ���ܵ�������_________________________��
(3)ʵ����̡�
�����Ӻ�ʵ��װ��________��Ȼ��Ƶ�Cװ�õ�����Ϊyg��
�ڳ�ȡxg AlN��Ʒ����Aװ����;���ý������ر�________��________(�K1����K2��);ͨ����Һ©������NaOHŨ��Һ�������ٲ������塣��K1��ͨ�뵪��һ��ʱ�䣬�Ƶ�Cװ�õ�������Ϊzg��ͨ�뵪����Ŀ����__________________��װ��B��������__________________________��
(4)���ݷ�����
��AlN����������Ϊ________��(���ԭ������Al��27��N��14)
������װ�ô���ȱ�ݣ����²ⶨ���ƫ�ߣ�������Ľ����________________��
������(1)AlN+NaOH+H2ONaAlO2+NH3��
(2)��ֹ����
(3)�ټ���װ�õ�������
��K1 K2 ��װ���в����İ���ȫ������Cװ�ã�ʹ������������� ���ﰱ��
(4)��![]() ��100%
��100%
��������(1)������Ŀ��Ϣ��AlN����ǿ����Һʱ������NH3����ѧ����ʽΪ��AlN+NaOH+H2ONaAlO2+NH3����
(2)������������ˮ��Ũ���ᷢ����Ӧ��Cװ�������θ�����ϲ�����ϴ���Է�ֹ�������������Σ�գ����²ⶨ�����ȷ��
(3)������װ���������壬Ӧ�ȼ���װ�õ������ԣ�Ȼ��Ƶ�Cװ�õ�����ΪygΪ���հ���������;
��������NaOH��Һ����Ʒ��AlN��ȫ��Ӧ������������ɵİ���������������������Ӧ�رջ���K1������K2��ʹ���ɵİ�����Ũ����ȫ�����գ�ͨ����Һ©������NaOHŨ��Һ�������ٲ������塣��K1��ͨ�뵪��һ��ʱ�䣬�Ƶ�Cװ�õ�������Ϊzg��ͨ�뵪����Ŀ���ǰ�װ���в����İ���ȫ������Cװ�ã�ʹ������������գ����ⰱ���е�ˮ����Ӱ��ⶨ�����ͨ��װ��B�еļ�ʯ������ˮ�������������и��
(4)�ٰ���������Ϊ(z-y)g�����ʵ���Ϊ![]() mol�����ݵ�ԭ�ӵ��غ㣬���������ʵ�������AlN�����ʵ���������AlN������Ϊ
mol�����ݵ�ԭ�ӵ��غ㣬���������ʵ�������AlN�����ʵ���������AlN������Ϊ![]() mol��41 g��mol-1=
mol��41 g��mol-1=![]() g����AlN����������Ϊ
g����AlN����������Ϊ![]() ��100%=
��100%=![]() ��100%;
��100%;
��װ��C��Ũ��������տ����е�ˮ����ʹ�ⶨ�İ����������Ľ������Cװ�ó��ڴ�����һ������װ�ã��Է�ֹ�����е�ˮ�������롣

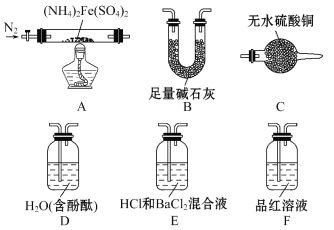
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
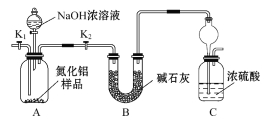
Сѧ��10����Ӧ����ϵ�д�����Ŀ����Al����ͬ��ͭ����Һ��ķ�ӦΪʵ�����̽��Al����ͬͭ����Һ��Ӧ�Ķ����ԡ�
ʵ�� | ���Թ��м���2ml��Һ | ʵ������ |
| ʵ���0.5mol/LCuCl2��Һ | 15s�������������к�ɫ�������� 60s����Ӧ��һ���ӿ��ҷ�Ӧ���ҷ��ȣ�Һ�弸������ 120s����Ƭ��Ӧ�꣬�Թ��������������ɵĺ�ɫ���� |
ʵ���0.5mol/LCuSO4��Һ | 15s ���������� 60s����Ƭ�����м������� 120s�������������������Ƭ��Ե�к��ٺ�ɫ�������� |
��1����ʵ��������о��ڢ�
��ʵ���������ɺ�ɫ���ʵ����ӷ���ʽΪ��____________________________
������ʵ����в���������Ϊ�����������ӷ���ʽ������Һ�д���H+��ԭ��_____________________
����д��60s��Ӧ��һ���ӿ���ܵ�ԭ��д��������_____________________________
(2)��ʵ�������о�
ʵ���Ӧ���Ա�ʵ�������˵��Al�벻ͬ��ͭ����Һ��Ӧ���ֶ����ԣ���ԭ������У�
����һ��SO42-��Al��Cu2+���û���Ӧ����һ�����������á�
�������________________________
(3)Ϊ��һ���о��������裬�������ʵ�飺
ʵ�� | ���Թ��м���2mL��Һ | ʵ������ |
| ʵ���0.5mol/LCuCl2��Һ+1g Na2SO4���� | ���� Na2SO4���������ʼ������䣬�����������ݲ�������ɫ���������Ҿ��ҷ��ȣ���Ƭ��Ӧ��ȫ����Һ���� |
ʵ�����0.5mol/L CuCl2��Һ+5g Na2SO4���� | ����Na2SO4���������ʼ������䣬�����������ݲ�������ɫ���������Ҿ��ҷ��ȣ���Ƭ��Ӧ��ȫ����Һ���� | |
ʵ�����0.5mol/LCuSO4��Һ+0.02mol NaCl���� | δ���� NaCl����ǰ����û�������������Ƭ����Ѹ�ٲ�����������ͺ�ɫ���ʣ���Һ�¶�����������������Ӧ��ȫ | |
ʵ�����0.5mol/LCuSO4��Һ+_________ NH4Cl���� | δ���� NH4Cl����ǰ����û�������������Ƭ����Ѹ�ٲ�����������ͺ�ɫ���ʣ���Һ�¶�����������������Ӧ��ȫ |
_____________________________________
������ʵ������ʵ�������Աȣ�����Ϊ��______________________________________
������ʵ������ʵ�����������Աȣ�����Ϊ��______________________________________
��4�������ʵ��֤��Cl-��Al��H+����Ӧͬ�������Ƶ����ã�_________________