��Ŀ����
��18�֣��Ͼ�Ӳ�ʺϽ��к�̼����(WC)��������(Co)�����������������õ�ⷨ����WC���Ʊ�CoxOy���Ĺ������̼�ͼ���£�
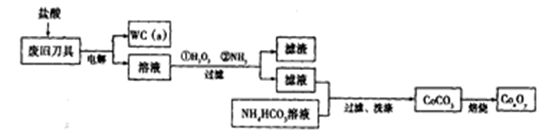
��1�����ʱ�Ͼɵ�����������������������������������______��
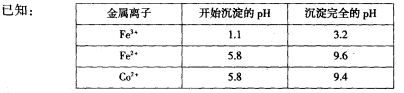
��2��ͨ�˰�����Ŀ���ǵ�����Һ��pH����ȥ��Ԫ�ء��ɱ��е����ݿ�֪�������Ͽ�ѡ��pH���Χ��____________��
��3��ʵ����NH4HCO3��Һ�Լ��ԣ��Ʊ�CoCO3ʱ��ѡ�õļ��Ϸ�ʽ��_______������ţ���ԭ����_______��
a������Һ��NH4HCO3��Һͬʱ���뵽��Ӧ������
b������Һ�������뵽ʢ��NH4HCO3��Һ�ķ�Ӧ������
c����NH4HCO3��Һ�������뵽ʢ����Һ�ķ�Ӧ������
д������CoCO3�����ӷ���ʽ______________________________________��
��4��ʵ���л�õ���ϴ�Ӳ���֣��ڱ���ʱ�������Ⱦ�����壬����Ⱦ������ijɷ�Ϊ_______________���ѧʽ����
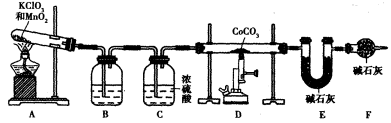
��5��ʵ����������װ����ȡ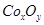 �����ⶨ�仯ѧʽ��
�����ⶨ�仯ѧʽ��
��װ��A�Ƶõ��к�������Cl2����װ��B����ʢ�ŵ��Լ�Ϊ______������ţ���
a��NaHCO3��Һ b��NaOH��Һ c��KMnO4��Һ d������NaCI��Һ
����CoCO3��ȫת��Ϊ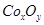 �����Ƶ�E������4��40g��D���ڲ������ʵ�������8��30g����������
�����Ƶ�E������4��40g��D���ڲ������ʵ�������8��30g����������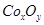 �Ļ�ѧʽΪ____________��
�Ļ�ѧʽΪ____________��
����ȱ��װ��F������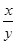 ��ֵ______���ƫ����ƫС������Ӱ�족����
��ֵ______���ƫ����ƫС������Ӱ�족����


��1�����ʱ�Ͼɵ�����������������������������������______��
��2��ͨ�˰�����Ŀ���ǵ�����Һ��pH����ȥ��Ԫ�ء��ɱ��е����ݿ�֪�������Ͽ�ѡ��pH���Χ��____________��
��3��ʵ����NH4HCO3��Һ�Լ��ԣ��Ʊ�CoCO3ʱ��ѡ�õļ��Ϸ�ʽ��_______������ţ���ԭ����_______��
a������Һ��NH4HCO3��Һͬʱ���뵽��Ӧ������
b������Һ�������뵽ʢ��NH4HCO3��Һ�ķ�Ӧ������
c����NH4HCO3��Һ�������뵽ʢ����Һ�ķ�Ӧ������
д������CoCO3�����ӷ���ʽ______________________________________��
��4��ʵ���л�õ���ϴ�Ӳ���֣��ڱ���ʱ�������Ⱦ�����壬����Ⱦ������ijɷ�Ϊ_______________���ѧʽ����
��5��ʵ����������װ����ȡ
 �����ⶨ�仯ѧʽ��
�����ⶨ�仯ѧʽ��
��װ��A�Ƶõ��к�������Cl2����װ��B����ʢ�ŵ��Լ�Ϊ______������ţ���
a��NaHCO3��Һ b��NaOH��Һ c��KMnO4��Һ d������NaCI��Һ
����CoCO3��ȫת��Ϊ
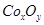 �����Ƶ�E������4��40g��D���ڲ������ʵ�������8��30g����������
�����Ƶ�E������4��40g��D���ڲ������ʵ�������8��30g����������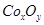 �Ļ�ѧʽΪ____________��
�Ļ�ѧʽΪ____________������ȱ��װ��F������
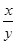 ��ֵ______���ƫ����ƫС������Ӱ�족����
��ֵ______���ƫ����ƫС������Ӱ�족����(l)���������Һ����2��3.2��pH��5.8��
��3��c����ֹ�����������ܳ�����Co2++2HCO3�� CoCO3��+CO2��+H2O����4��HCl��NH3����5����b����Co2O3����ƫ��
CoCO3��+CO2��+H2O����4��HCl��NH3����5����b����Co2O3����ƫ��
��3��c����ֹ�����������ܳ�����Co2++2HCO3��
 CoCO3��+CO2��+H2O����4��HCl��NH3����5����b����Co2O3����ƫ��
CoCO3��+CO2��+H2O����4��HCl��NH3����5����b����Co2O3����ƫ������������������������֪��������Ϊ�������Һ���ԷϾɵ���������������������������е�⣬����WC����Һ�к����������Ӻ�Co2+������˫��ˮ����������������Ϊ�����ӣ�ͨ�백��������pH��3.2��5.8֮�䣬��������ת��Ϊ���������������˳�ȥ����Һ�к���Co2+������̼�������Һ����̼���ܣ���������CoxOy��(l)���ʱ�Ͼɵ��������������������������������������������Һ����2��ͨ�˰�����Ŀ���ǵ�����Һ��pH����ȥ��Ԫ�ء��ɱ��е����ݿ�֪�������Ͽ�ѡ��pH���Χ��3.2��pH��5.8����3��ʵ����NH4HCO3��Һ�Լ��ԣ��Ʊ�CoCO3ʱ��Ϊ��ֹ�����������ܳ�����ѡ�õļ��Ϸ�ʽ��c����NH4HCO3��Һ�������뵽ʢ����Һ�ķ�Ӧ�����С�����CoCO3�����ӷ���ʽΪCo2++2HCO3��
 CoCO3��+CO2��+H2O����4��ʵ���л�õ���ϴ�Ӳ���ֻẬ���Ȼ�����ʣ��ڱ���ʱ�Ȼ�立ֽ�������Ⱦ������HCl��NH3����5�������װ��ͼ��֪��ʵ��ͨ���ⶨװ��E�е����أ�ȷ�����ɵĶ�����̼��������������n��C��=n��Co����֪CoxOy��Co�����ʵ�������������Co��������װ��D���ڲ���������CoxOy���������Ԫ���������ټ�����ԭ�ӵ����ʵ���������ԭ�����ʵ���֮��ȷ����ѧʽ����װ��A���Ƶõ�O2�к���������Cl2��Bװ������ʢ�ŵ�������������Cl2��a������NaHCO3��Һ�������������������ɶ�����̼��Ӱ�������̼�����IJⶨ������b��NaOH��Һ�������������������������䣬��ȷ��c��c��KMnO4��Һ������������������d������NaCl��Һ������������������ѡb����E������4.40g�Ƕ�����̼�����������ʵ���Ϊ0.1mol�����ݻ�ѧʽCoCO3��֪��n��Co��=n��C��=0.1mol��Co������Ϊ0.1mol��59g/mol=5.9g��D���ڲ������ʵ�����8.30g��CoxOy��������CoxOy����Ԫ������Ϊ8.3g-5.9g=2.4g����ԭ�ӵ����ʵ���n��O��=0.15mol��n��Co����n��O��=0.1mol��0.15mol=2��3�����ܵ�������Ļ�ѧʽΪCo2O3����ȱ��Fװ�ã�װ��E�м�ʯ�ҿ������տ�����ˮ������������̼����ɶ�����̼����ƫ����Co������ƫ��x/y��ֵƫ��
CoCO3��+CO2��+H2O����4��ʵ���л�õ���ϴ�Ӳ���ֻẬ���Ȼ�����ʣ��ڱ���ʱ�Ȼ�立ֽ�������Ⱦ������HCl��NH3����5�������װ��ͼ��֪��ʵ��ͨ���ⶨװ��E�е����أ�ȷ�����ɵĶ�����̼��������������n��C��=n��Co����֪CoxOy��Co�����ʵ�������������Co��������װ��D���ڲ���������CoxOy���������Ԫ���������ټ�����ԭ�ӵ����ʵ���������ԭ�����ʵ���֮��ȷ����ѧʽ����װ��A���Ƶõ�O2�к���������Cl2��Bװ������ʢ�ŵ�������������Cl2��a������NaHCO3��Һ�������������������ɶ�����̼��Ӱ�������̼�����IJⶨ������b��NaOH��Һ�������������������������䣬��ȷ��c��c��KMnO4��Һ������������������d������NaCl��Һ������������������ѡb����E������4.40g�Ƕ�����̼�����������ʵ���Ϊ0.1mol�����ݻ�ѧʽCoCO3��֪��n��Co��=n��C��=0.1mol��Co������Ϊ0.1mol��59g/mol=5.9g��D���ڲ������ʵ�����8.30g��CoxOy��������CoxOy����Ԫ������Ϊ8.3g-5.9g=2.4g����ԭ�ӵ����ʵ���n��O��=0.15mol��n��Co����n��O��=0.1mol��0.15mol=2��3�����ܵ�������Ļ�ѧʽΪCo2O3����ȱ��Fװ�ã�װ��E�м�ʯ�ҿ������տ�����ˮ������������̼����ɶ�����̼����ƫ����Co������ƫ��x/y��ֵƫ��
��ϰ��ϵ�д�
�����Ŀ
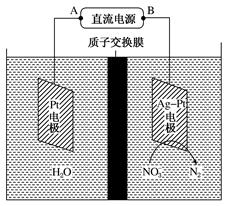
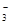 ��6H2O��10e��=N2����12OH��
��6H2O��10e��=N2����12OH��