��Ŀ����
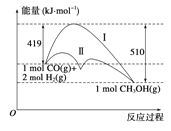
����Ŀ����֪ ��H��(aq)��OH��(aq)=H2O(l) ��H1�� -57.3 kJ��mol��1��
��2H2(g)��O2(g)=2H2O(l) ��H2�� -571.6 kJ��mol��1������˵����ȷ����
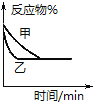
A.��0.1 mol NaOH����Һ�м���һ�����0.1 mol��L��1�Ҷ��ᣨHOOC-COOH)����Ӧ�е������仯��ͼ��ʾ
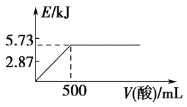
B.������ȼ����Ϊ571.6 kJ��mol��1
C.����Ӧ����ˮΪ��̬����ͬ�������µķ�Ӧ�ȣ���H<��H2
D.NH3��H2O(aq)��H��(aq)=NH4+ (aq)��H2O(l) ��H����57.3 kJ��mol��1
���𰸡�A
��������
A�0.1 mol NaOH����Һ��500ml0.1 mol��L��1�Ҷ���ǡ����ȫ��Ӧ����0.1molˮ�������Ҷ���Ϊ��Ԫ���ᣬ����Һ�еĵ���Ϊ���ȹ��̣���Ӧ�ų�������С��5.73 kJ����A��ȷ��
B�ȼ����Ϊ1mol������ȫȼ�������ȶ�������ų�����������������ȼ����Ϊ285.8kJ��mol��1����B����
C���̬ˮת��ΪҺ̬ˮ�ų�����������Ӧ����ˮΪ��̬���ų����������ڷ�Ӧ�����Ƚ��ʱ�ʱҪ������������Ӧ����H2<��H����C����
D�NH3��H2OΪ�������Һ�еĵ���Ϊ���ȹ��̣���Ӧ����H����57.3 kJ��mol��1����D����
��ѡA��

����Ŀ���������ӷ���ʽ����д�����ۣ�����������
ѡ�� | ���ӷ���ʽ | ���� |
A | ��ͭ�缫��ⱥ��KCl��Һ��2H2O+2Cl- | ��ȷ��Cl-��ʧ����������OH-ǿ |
B | ��CuSO4��Һ��ͨ�������H2S���壺Cu2++H2S=CuS��+2H+ | ����H2S�����Ա�H2SO4�� |
C | Ba(HCO3)2��Һ��������NaOH��Һ��Ӧ��Ba2++HCO3- +OH- �TBaCO3��+H2O | ����Ba2+��HCO3-ϵ����ӦΪ1:2 |
D | ����SO2ͨ�뵽NaClO��Һ�У�SO2+ClO- +H2O= HClO+HSO3- | ��ȷ��H2SO3�����Ա�HClOǿ |
A.AB.BC.CD.D