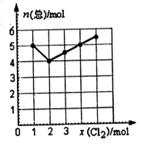
��Ŀ����
��18�֣�ij��γ�С������50mLNaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ���CO2�����������NaHCO3�����������ʵ�鲽�裺
a.ȡ25 mL NaOH��Һ���չ�����CO2���壬��CO2���岻���ܽ⣻
b.С�������Һ1��2 min��
c.�ڵõ�����Һ�м�����һ��(25mL)NaOH��Һ��ʹ���ֻ�Ϸ�Ӧ��
(1)�˷������Ƶýϴ�����Na2CO3��д��a��c�����Ļ�ѧ��Ӧ����____________________��____________________________��
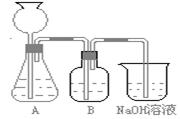
�˷�����һ����ʵ��װ������ͼ��ʾ��
(2)���뷴Ӧ��ǰ����μ������װ�õ�������______________________________________��
(3)װ��B��ʢ�ŵ��Լ���______________��
������___________________ ____________��
(4)��ʵ����ͨ���Ʒ��У�װ��A������Ϊ����_____________ ����ķ���װ��(�����)��
��CH2==CH2 ��H2S ��CH4 ��CH��CH ��H2
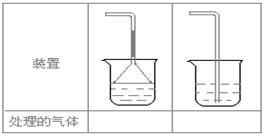
 (5)ʵ������ȡ�������壺��NH3����Cl2����HCl����H2S����CH4����CO����CO2����O2ʱ�����ڱ������β����������������ͼ��ʾװ�ý��д����ģ���������������װ��ͼ���·��ո��ڡ�
(5)ʵ������ȡ�������壺��NH3����Cl2����HCl����H2S����CH4����CO����CO2����O2ʱ�����ڱ������β����������������ͼ��ʾװ�ý��д����ģ���������������װ��ͼ���·��ո��ڡ�
(6)��֪����NaOH��Һ�����ʵ���������Ϊ40%�������¸���Һ�ܶ�Ϊ1.44 g / mL�����跴Ӧǰ����Һ��������䣬������ʵ���������ô��ַ����Ʊ�����Na2CO3��Һ�����ʵ���Ũ��Ϊ____________________��

a.ȡ25 mL NaOH��Һ���չ�����CO2���壬��CO2���岻���ܽ⣻
b.С�������Һ1��2 min��
c.�ڵõ�����Һ�м�����һ��(25mL)NaOH��Һ��ʹ���ֻ�Ϸ�Ӧ��
(1)�˷������Ƶýϴ�����Na2CO3��д��a��c�����Ļ�ѧ��Ӧ����____________________��____________________________��
�˷�����һ����ʵ��װ������ͼ��ʾ��
(2)���뷴Ӧ��ǰ����μ������װ�õ�������______________________________________��
(3)װ��B��ʢ�ŵ��Լ���______________��
������___________________ ____________��
(4)��ʵ����ͨ���Ʒ��У�װ��A������Ϊ����_____________ ����ķ���װ��(�����)��
��CH2==CH2 ��H2S ��CH4 ��CH��CH ��H2
 (5)ʵ������ȡ�������壺��NH3����Cl2����HCl����H2S����CH4����CO����CO2����O2ʱ�����ڱ������β����������������ͼ��ʾװ�ý��д����ģ���������������װ��ͼ���·��ո��ڡ�
(5)ʵ������ȡ�������壺��NH3����Cl2����HCl����H2S����CH4����CO����CO2����O2ʱ�����ڱ������β����������������ͼ��ʾװ�ý��д����ģ���������������װ��ͼ���·��ո��ڡ�
(6)��֪����NaOH��Һ�����ʵ���������Ϊ40%�������¸���Һ�ܶ�Ϊ1.44 g / mL�����跴Ӧǰ����Һ��������䣬������ʵ���������ô��ַ����Ʊ�����Na2CO3��Һ�����ʵ���Ũ��Ϊ____________________��
(1) 2NaOH + CO2 ="=" NaHCO3 NaHCO3 + NaOH ="=" Na2CO3 + H2O
(2)�����٣��õ��ɼм�סA��B���Ӵ����ȼ��A�������ԣ�������Ƥ������©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬ֹͣ��ˮ��©��������ƿ�е�Һ���ֲ��䣬˵��װ�ò�©����Ȼ����B�������ԣ����ձ���ע������ˮ��ʹ���ܿ�����ˮ�У�˫����ס���ƿƬ��������ð�����ɿ��ֺ�������ˮ���뵼���γ�ˮ����˵��װ�ò�©����
�����ڣ�Ҳ��һ�μ��A��B�������ԣ����Ӻ��ձ�����齺����ֹˮ�м�ס��Ȼ���©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬��һ�ᣬ�۲�©��������ƿ�е�Һ�������ֲ��䣬˵��װ�ò�©����
(3) ������������Һ ����HCl����
(4) �ڢܢ� (5) �٢� �ڢ� (6) 7.2 mol/L
(2)�����٣��õ��ɼм�סA��B���Ӵ����ȼ��A�������ԣ�������Ƥ������©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬ֹͣ��ˮ��©��������ƿ�е�Һ���ֲ��䣬˵��װ�ò�©����Ȼ����B�������ԣ����ձ���ע������ˮ��ʹ���ܿ�����ˮ�У�˫����ס���ƿƬ��������ð�����ɿ��ֺ�������ˮ���뵼���γ�ˮ����˵��װ�ò�©����
�����ڣ�Ҳ��һ�μ��A��B�������ԣ����Ӻ��ձ�����齺����ֹˮ�м�ס��Ȼ���©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬��һ�ᣬ�۲�©��������ƿ�е�Һ�������ֲ��䣬˵��װ�ò�©����
(3) ������������Һ ����HCl����
(4) �ڢܢ� (5) �٢� �ڢ� (6) 7.2 mol/L
��

��ϰ��ϵ�д�
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
�����Ŀ