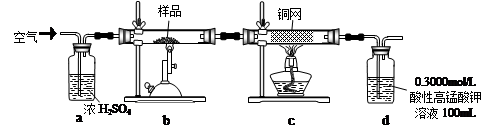
��Ŀ����
��16�֣���һ��ijͬѧ��һֻ�ձ���װ��һ�����Ĵ����ۣ�����200mL 6mol/L�����ᣬ����ǡ���ܽ⣬��̽����������Ԫ�ؼ�̬��
(1)������裺
����1������ֻ��+2������
����2:___________________________________��
����3:___________________________________��
(2)���ʵ�飺ȡ��Ӧ������Һ�ֱ�װ��ס�����֧�Թܣ��ڼ��еμ�����KMnO4��Һ�������еμ�KSCN��Һ���۲������Ʋ�ʵ����������ۣ�
��������Ϊ____________________�������1��ȷ��
��������Ϊ____________________�������2��ȷ��
��������Ϊ____________________�������3��ȷ��
���������Ȼ����dz�����ˮ����������ҵ���Ʊ���ˮFeCl3������Ϊ��
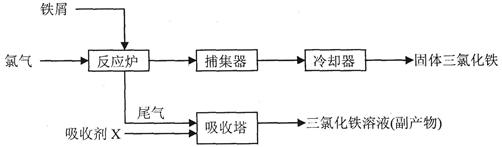
(3)���ռ�XΪFeCl2��Һ������β��Cl2��Ӧ�����ӷ���ʽ__________________��
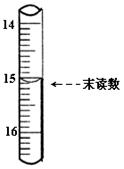
(4)��ȡ������Ʒm������25mLϡ���ᣬ������ˮ���50mL��Һ�������Թ�����KI��Һ��ַ�Ӧ��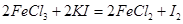 ���õ�����ָʾ������
���õ�����ָʾ������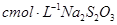 ��Һ���еζ�
��Һ���еζ�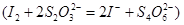 ������Na2S2O3��ҺVmL������Ʒ��FeCl3����������Ϊ____��(���ԭ������Fe��56 Cl��35.5)
������Na2S2O3��ҺVmL������Ʒ��FeCl3����������Ϊ____��(���ԭ������Fe��56 Cl��35.5)
(5)��FeCl3��Һ(32%-35%)��ʴͭ���·ʱ���÷�Һ��FeCl3��FeCl2��CuCl2�����û�ѧ�������շ�Һ�е�ͭ����������Ҫ�㣺___________________________________________________��
(1)������裺
����1������ֻ��+2������
����2:___________________________________��
����3:___________________________________��
(2)���ʵ�飺ȡ��Ӧ������Һ�ֱ�װ��ס�����֧�Թܣ��ڼ��еμ�����KMnO4��Һ�������еμ�KSCN��Һ���۲������Ʋ�ʵ����������ۣ�
��������Ϊ____________________�������1��ȷ��
��������Ϊ____________________�������2��ȷ��
��������Ϊ____________________�������3��ȷ��
���������Ȼ����dz�����ˮ����������ҵ���Ʊ���ˮFeCl3������Ϊ��

(3)���ռ�XΪFeCl2��Һ������β��Cl2��Ӧ�����ӷ���ʽ__________________��
(4)��ȡ������Ʒm������25mLϡ���ᣬ������ˮ���50mL��Һ�������Թ�����KI��Һ��ַ�Ӧ��
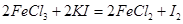 ���õ�����ָʾ������
���õ�����ָʾ������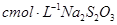 ��Һ���еζ�
��Һ���еζ�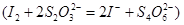 ������Na2S2O3��ҺVmL������Ʒ��FeCl3����������Ϊ____��(���ԭ������Fe��56 Cl��35.5)
������Na2S2O3��ҺVmL������Ʒ��FeCl3����������Ϊ____��(���ԭ������Fe��56 Cl��35.5)(5)��FeCl3��Һ(32%-35%)��ʴͭ���·ʱ���÷�Һ��FeCl3��FeCl2��CuCl2�����û�ѧ�������շ�Һ�е�ͭ����������Ҫ�㣺___________________________________________________��
(1)����2��������ֻ��+3����Ԫ�أ�1�֣�
����3�������м���+2������+3����Ԫ�ء���1�֣�
(2)�ټ��Թ���Һ��ɫ��ȥ�����Թ�û�����Ա仯����2�֣�
�ڼ��Թ���Һ�����Ա仯�����Թ���Һ���ɫ����2�֣�
�ۼ��Թ���Һ��ɫ��ȥ�����Թ���Һ���ɫ����2�֣�
(3)Cl2��2Fe2��=2Cl����2Fe3����3�֣�
(4)16.25 Vc/m%��3�֣�
(5)�ӹ���Fe�ۣ������ù����ù��������ܽ���������ۣ����ˡ�ϴ�ӡ������2�֣�
����3�������м���+2������+3����Ԫ�ء���1�֣�
(2)�ټ��Թ���Һ��ɫ��ȥ�����Թ�û�����Ա仯����2�֣�
�ڼ��Թ���Һ�����Ա仯�����Թ���Һ���ɫ����2�֣�
�ۼ��Թ���Һ��ɫ��ȥ�����Թ���Һ���ɫ����2�֣�
(3)Cl2��2Fe2��=2Cl����2Fe3����3�֣�
(4)16.25 Vc/m%��3�֣�
(5)�ӹ���Fe�ۣ������ù����ù��������ܽ���������ۣ����ˡ�ϴ�ӡ������2�֣�
��1���������ļ�̬�ǣ�2�ۻ�3�ۣ����Էֱ��Ǽ���2��������ֻ��+3����Ԫ�أ�����3�������м���+2������+3����Ԫ�ء�
(2)�������������Ӿ��л�ԭ�ԣ������������I��ȷ���������Ǽ��Թ���Һ��ɫ��ȥ�����Թ�û�����Ա仯��
������Ǽ������ȷ�����������ܺ�KSCN��Һ������ɫ��Ӧ����������Ǽ��Թ���Һ�����Ա仯�����Թ���Һ���ɫ��
������Ǽ������ȷ����������Ǽ��Թ���Һ��ɫ��ȥ�����Թ���Һ���ɫ��
��3���������������ԣ��������Ȼ������������Ȼ���������ʽΪCl2��2Fe2��=2Cl����2Fe3����
��4�����ݷ���ʽ��֪��FeCl3��Na2S2O3�������Ȼ��������ʵ�����0.001cVmol��������Ʒ��FeCl3����������Ϊ ��
��
(5)Ҫ����ͭ����Ӧ�ü�����������ۣ��û���ͭ�����������������ܽ⼴�ɡ�������ȷ�IJ����Ǽӹ���Fe�ۣ������ù����ù��������ܽ���������ۣ����ˡ�ϴ�ӡ����
�������ۣ����ˡ�ϴ�ӡ������2�֣�
(2)�������������Ӿ��л�ԭ�ԣ������������I��ȷ���������Ǽ��Թ���Һ��ɫ��ȥ�����Թ�û�����Ա仯��
������Ǽ������ȷ�����������ܺ�KSCN��Һ������ɫ��Ӧ����������Ǽ��Թ���Һ�����Ա仯�����Թ���Һ���ɫ��
������Ǽ������ȷ����������Ǽ��Թ���Һ��ɫ��ȥ�����Թ���Һ���ɫ��
��3���������������ԣ��������Ȼ������������Ȼ���������ʽΪCl2��2Fe2��=2Cl����2Fe3����
��4�����ݷ���ʽ��֪��FeCl3��Na2S2O3�������Ȼ��������ʵ�����0.001cVmol��������Ʒ��FeCl3����������Ϊ
 ��
��(5)Ҫ����ͭ����Ӧ�ü�����������ۣ��û���ͭ�����������������ܽ⼴�ɡ�������ȷ�IJ����Ǽӹ���Fe�ۣ������ù����ù��������ܽ���������ۣ����ˡ�ϴ�ӡ����
�������ۣ����ˡ�ϴ�ӡ������2�֣�

��ϰ��ϵ�д�
�����Ŀ
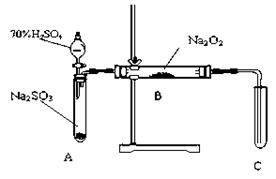
 �������װ�øĽ��Ĵ�ʩ�����ɣ�
�������װ�øĽ��Ĵ�ʩ�����ɣ�




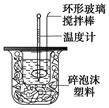
 2Fe2O3+8SO2����������ʵ�飬���ⶨ����Ʒ��FeS2��Ʒ�Ĵ��ȣ������������ʲ����뷴Ӧ����
2Fe2O3+8SO2����������ʵ�飬���ⶨ����Ʒ��FeS2��Ʒ�Ĵ��ȣ������������ʲ����뷴Ӧ����