��Ŀ����
��10�֣���1�������£���20.0g14%��NaCl��Һ��30.0g24%��NaCl��Һ��ϣ���Ϻ�õ��ܶ�Ϊ1.17g/cm3����Һ������㣺��������̲��÷֣�
�ٻ�Ϻ���Һ��NaCl����������
�ڻ�Ϻ���Һ��NaCl�����ʵ���Ũ��
����1000gˮ�мӶ��� mol NaCl������ʹ��Ũ��ǡ����������Ϻ���Һ��Ũ����ȡ����������һλС����
��2����״���£�11.2LO2��CO2������������Ϊ19.6g������������O2��CO2�����֮��
��1���ٻ�Ϲ����������Dz����
���Ի�Ϻ���Һ��NaCl������������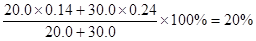
�ڸ���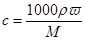 ��֪��Ϻ���Һ��NaCl�����ʵ���Ũ����
��֪��Ϻ���Һ��NaCl�����ʵ���Ũ����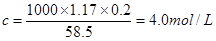
�۸�������������֪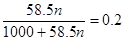 �����x��4.3mol
�����x��4.3mol
��2������������O2��CO2�����ʵ����ֱ���x��y
��״���£�11.2L����������ʵ�����11.2L��22.4L/mol��0.5mol
������x��y��0.5mol��32x��44y��19.6g
���x��0.2mol��y��0.3mol
���Ի��������O2��CO2�����֮����2:3
����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ