��Ŀ����
��20�֣�ij��ѧ����С������м��ϡH2SO4��NaOH��ҺΪ��Ҫԭ�����Ʊ�Al(OH)3������������������ַ��������±����Ķ��±����ش��������⣺
|
;�� |
����1 mol Al(OH)3����H+��OH�������ʵ���/mol |
|
|
����H+ |
����OH�� |
|
|
1��Al��Al3+��Al(OH)3 |
|
|
|
2��Al��AlO |
|
|
|
3�� |
|
|
��1����д�ϱ��пոӽ�Լԭ�ϵĽǶ�������������ΪӦѡ��_________��Ϊ������
��2����ʵ��Ҫ�õ�NaOH��Һ��ijѧ������֪����y(g)�ı�����ȷ��ȡ ��g��NaOH���塣����������ƽ�������Ϸ��루
��g��NaOH���塣����������ƽ�������Ϸ��루 + y��(g)���룬�����̵ı������м���NaOH���壬��ʱָ��ƫ���ұߣ���ͼ��ʾ���������IJ���Ӧ����_________ ʹ
��
+ y��(g)���룬�����̵ı������м���NaOH���壬��ʱָ��ƫ���ұߣ���ͼ��ʾ���������IJ���Ӧ����_________ ʹ
��

��3������ȡ�� (g)NaOH�պÿ�����0.5mol��L��1NaOH��Һ500mL��������������Һ����ʾ��ͼ���д�����ǣ��������ţ�______________��
(g)NaOH�պÿ�����0.5mol��L��1NaOH��Һ500mL��������������Һ����ʾ��ͼ���д�����ǣ��������ţ�______________��

��4���Ķ������Ʊ�Al(OH)3ʵ�鲽�裬��д�հף�
�����ձ�A�м���50mL0.5mol��L NaOH��Һ���ټ���������м������Һ�Լ��ȡ��������ǣ�__________________________������ˮ����м��ϴ�ɾ���������м������Ϊm1(g)��
NaOH��Һ���ټ���������м������Һ�Լ��ȡ��������ǣ�__________________________������ˮ����м��ϴ�ɾ���������м������Ϊm1(g)��
����ʢ������ϡH2SO4���ձ�B��Ӧ����___________��g�����ú�m1��ʽ�ӱ�ʾ����������м����ֽ���ʹ��м��Ӧ��ȫ��
����ʢ������ŨNaOH��Һ���ձ�C�з���____________��g�����ú�m1��ʽ�ӱ�ʾ����������м��ֽ���ʹ��м��Ӧ��ȫ��
�ܽ��ձ�B���ձ�C�е���Һ��۲쵽�������ǣ�_______________________________����Ӧ�����ӷ���ʽ�ǣ�___________________________
��5������ʱijѧ��������ͼ����������˵��ͼ�д�����ǣ�
_____________________ ��

��6���ѳ���ת�Ƶ��ձ��У�������ˮϴ�������ٹ��ˣ���ϴ�ӡ�
�����Al(OH)3��������Ϊm2(g)���㱾ʵ��Al(OH)3�IJ�����_________________
��1��
|
;�� |
����1 mol Al(OH)3����H+��OH�������ʵ���/mol |
|
|
����H+ |
����OH�� |
|
|
1 |
3 |
3 |
|
2 |
1 |
1 |
|
3 |
3/4 |
3/4 |
����3��ÿ��1�֣���7�֣�
��2������������Ʒ ָ��ָ�ڱ�ߵ��м䣨2�֣� ��3���٢ۢܢ� ��2�֣�
��4���ٳ�ȥ���۱����Al2O3����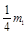 ����
���� ����3�֣������ɰ�ɫ��״���� 3AlO
����3�֣������ɰ�ɫ��״���� 3AlO
4Al(OH)3������2�֣���5���������¶�Ӧ�õ�����ֽ��Ե��©����û�У���Ӧ�ã������ձ��ڱ�.����2�֣�
��6��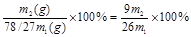 ��2�֣�
��2�֣�
����������1�����ݷ���ʽ2Al��6H��=2Al3��3H2����Al3����3OH��=Al(OH)3������2Al��2OH����2H2O=2AlO2����3H2����Al2����H����H2O=Al(OH)3����3AlO
4Al(OH)3�������ɼ�������ĵ������ӻ�OH�������ʵ������������ĵ�ԭ�Ϸ���������3����õġ�
��2��ָ��ƫ���ұߣ�˵����Ʒ������������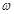 g������Ӧ�ü���������Ʒ��ʹָ��ָ�ڱ�ߵ��м䡣
g������Ӧ�ü���������Ʒ��ʹָ��ָ�ڱ�ߵ��м䡣
��3���������ʵ���Ũ����Һ�����ƣ��ܽ�Ӧ�����ձ��н��У��ٲ���ȷ��ת��ʹ����ͷ�ιܵ��¶�Ӧ�÷��ڿ̶��ߵ����棬�۲���ȷ���ܲ���ȷ����Ϊû��ϴ�ӣ��ݲ���ȷ������ʱӦ��ƽ�ӣ�
��ѡ�٢ۢܢݡ�
��4������Ƭ���溬������������Ҫ�������Ƴ�ȥ��
�ڸ���3AlO
4Al(OH)3����֪�����������ӵ����ĵ�����������AlO2�����ĵ�������֮����1�U3�ģ�����B��Ӧ����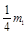 ������
������
�۸��ݢڿ�֪C�з��� ������
������
�������Ӻ�AlO2����Ӧ����������������������ʽΪ3AlO
4Al(OH)3����
��5������װ��ͼ��֪����2�����ֱ��Dz������¶�Ӧ�õ�����ֽ��Ե��©����û�У���Ӧ�ã������ձ��ڱڡ�
��6��m1g�����Ͽ��������������� �����Բ�����
�����Բ�����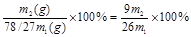 ��
��

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д� ��Al(OH)3
��Al(OH)3




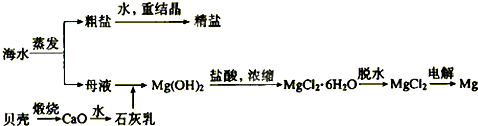
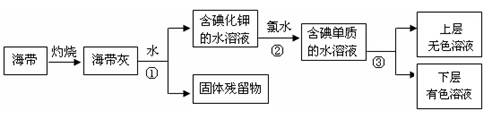

 �� ��NaOH+H2��+�� ���� ����
�� ��NaOH+H2��+�� ���� ����